Lesion to the nigrostriatal dopamine system disrupts stimulus-response habit formation - PubMed (original) (raw)
Comparative Study
Lesion to the nigrostriatal dopamine system disrupts stimulus-response habit formation
Alexis Faure et al. J Neurosci. 2005.
Abstract
Acquisition and performance of instrumental actions are assumed to require both action-outcome and stimulus-response (S-R) habit processes. Over the course of extended training, control over instrumental performance shifts from goal-directed action-outcome associations to S-R associations that progressively gain domination over behavior. Lesions of the lateral part of the dorsal striatum disrupt this process, and rats with lesions to the lateral striatum showed selective sensitivity to devaluation of the instrumental outcome (Yin et al., 2004), indicating that this area is necessary for habit formation. The present experiment further explored the basis of this dysfunction by examining the ability of rats subjected to bilateral 6-hydroxydopamine lesions of the nigrostriatal dopaminergic pathway to develop behavioral autonomy with overtraining. Rats were given extended training on two cued instrumental tasks associating a stimulus (a tone or a light) with an instrumental action (lever press or chain pull) and a food reward (pellets or sucrose). Both tasks were run daily in separate sessions. Overtraining was followed by a test of goal sensitivity by satiety-specific devaluation of the reward. In control animals, one action (lever press) was insensitive to reward devaluation, indicating that it became a habit, whereas the second action (chain pull) was still sensitive to goal devaluation. This result provides evidence that the development of habit learning may depend on the characteristics of the response. In dopamine-depleted rats, lever press and chain pull remained sensitive to reward devaluation, evidencing a role of striatal dopamine transmission in habit formation.
Figures
Figure 2.
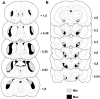
Diagrams of coronal sections (in reference to bregma) in the dorsal striatum (A) and the substantia nigra pars compacta (B) representing the extent of cell loss and dopaminergic deafferentation observed after bilateral infusions of 6-OHDA in the lateral dorsal striatum are shown. This histological reconstruction reveals the largest (Max; darker) and smallest (Min; lighter) regions of damage induced in the dorsal striatum (DA fibers loss) and substantia nigra (DA fibers and DA cell loss).
Figure 1.
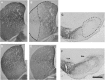
Microphotographs illustrating striatal DARPP-32 immunoreactivity (A, D) and TH immunoreactivity in the striatum (B, E), in the VTA and in the SNc (C-F) for the 6-OHDA-injected (A-C) and sham animals (D-F) are shown. Dashed lines in B and C represent an example of the lesion demarcation used for the reconstruction. Note that in the striatum of the 6-OHDA-injected animals, the DARPP-32 immunoreactivity does not reveal a noteworthy neuronal loss, whereas in the adjacent section, TH immunoreactivity reveals a drastic loss of dopaminergic processes. Accordingly, in the 6-OHDA-injected animals, the lateral part of the substantia nigra is nearly devoid of dopaminergic neurons. Scale bars (in E, F) A-F, 500 μm.
Figure 3.
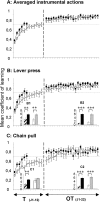
The mean coefficient of learning for averaged actions (A), the lever press (B), and the chain pull (C) is shown. The sham and 6-OHDA-injected animals' performances are represented by black squares and open circles, respectively, during the 12 d of training [T (J1-12)] and the 22 d of overtraining [OT (J1-22)]. A detailed representation of the mean rates of action before the stimulus (sham, hatched histograms and lesioned rats, white histograms) and during stimulus presentation (sham, black histograms and lesioned rats, gray histograms) for lever press (B1, B2) and chain pull (C1, C2) during the criterion session (B1, C1) and the last overtraining session (B2, C2) is shown. Note that the 6-OHDA-injected animals needed 6 more days to reach the global criterion (both actions) than the sham animals. Moreover, 6-OHDA-injected animals reached the criterion only on the 12th day of learning for the lever press, whereas they reached the criterion on the fourth day of overtraining for the chain pull. Nevertheless, even if 6-OHDA-injected animals were impaired in the learning of the task, there is no more effect of 6-OHDA-induced DA depletion on the overtrained performances. Data are expressed as means ± SEM.
Figure 4.
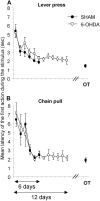
The mean latency of the first action to the stimulus for the lever press (A) and the chain pull (B) is shown. On the graph, the training and the last day of overtraining (OT) are represented. Note that the striatal 6-OHDA injection did not modify the latency of the first lever press or chain pull. Data are expressed as means ± SEM.
Figure 5.
The mean number of eaten pellets during satiety tests after extinction (A, B) and after reward (C, D) for the devalued lever press group (A, C) and the devalued chain pull group (B, D) is shown. Gray and black bars represent the mean number of satiated pellets consumed by the 6-OHDA-injected and the sham groups, respectively. Open bars represent the mean number of satiated eaten pellets in sham and 6-OHDA-injected rats. Note that whatever the conditions, the number of satiated eaten pellets was significantly lower that the mean number of nonsatiated eaten pellets. Moreover, there was no significant difference between sham and 6-OHDA-injected animals. Data are expressed as means ± SEM. *p < 0.05; **p < 0.01.
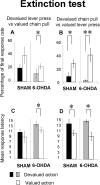
Figure 6.
Extinction test. The mean percentage of final response rate during the stimulus (A, B) and mean latency of the first action to the stimulus (C, D) for the first six stimuli for the devalued lever press group (A, C) and the devalued chain pull group (B, D) are shown. Gray and black bars represent the mean percentage or latency of devalued action for the 6-OHDA-injected and sham groups, respectively. White bars represent the mean percentage or latency of nondevalued action in sham and 6-OHDA-injected rats. Note that the lever press is not sensitive to outcome devaluation for sham animal either on the rate of action or on their latency. In contrast, for 6-OHDA-injected animals, both mean percentage of lever press and latency show sensitivity to the outcome devaluation. In contrast, the chain pull (percentage or latency) is sensitive to the outcome devaluation in sham as in 6-OHDA-injected animals. Data are expressed as means ± SEM. *p < 0.05; **p < 0.01.
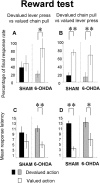
Figure 7.
Reward test. The mean percentage of the final response rate during the stimulus (A, B) and mean latency of the first action to the stimulus (C, D) for the devalued lever press group (A, C) and the devalued chain pull group (B, D) are shown. Gray and black bars represent the mean percentage or latency of the devalued action for the 6-OHDA-injected and sham groups, respectively. Open bars represent the mean percentage or latency of nondevalued action in sham and 6-OHDA-injected rats. Note that the lever press is not sensitive to outcome devaluation for sham animal either on the percentage or on the latency. In contrast, for 6-OHDA-injected animals, the mean percentage of lever press to the stimulus and latency are sensitive to the outcome devaluation. In contrast, the chain pull (percentage or latency) is sensitive to the outcome devaluation in sham as in 6-OHDA-injected animals. Data are expressed as means ± SEM. *p < 0.05; **p < 0.01.
Similar articles
- Dopamine agonists increase perseverative instrumental responses but do not restore habit formation in a rat model of Parkinsonism.
Faure A, Leblanc-Veyrac P, El Massioui N. Faure A, et al. Neuroscience. 2010 Jun 30;168(2):477-86. doi: 10.1016/j.neuroscience.2010.03.047. Epub 2010 Apr 1. Neuroscience. 2010. PMID: 20362642 - Amygdala central nucleus interacts with dorsolateral striatum to regulate the acquisition of habits.
Lingawi NW, Balleine BW. Lingawi NW, et al. J Neurosci. 2012 Jan 18;32(3):1073-81. doi: 10.1523/JNEUROSCI.4806-11.2012. J Neurosci. 2012. PMID: 22262905 Free PMC article. - The role of dopamine in the prelimbic cortex and the dorsomedial striatum in instrumental conditioning.
Lex B, Hauber W. Lex B, et al. Cereb Cortex. 2010 Apr;20(4):873-83. doi: 10.1093/cercor/bhp151. Epub 2009 Jul 15. Cereb Cortex. 2010. PMID: 19605519 - Habit formation: implications for alcoholism research.
O'Tousa D, Grahame N. O'Tousa D, et al. Alcohol. 2014 Jun;48(4):327-35. doi: 10.1016/j.alcohol.2014.02.004. Epub 2014 Apr 19. Alcohol. 2014. PMID: 24835007 Free PMC article. Review. - What Role Does Striatal Dopamine Play in Goal-directed Action?
Hart G, Burton TJ, Balleine BW. Hart G, et al. Neuroscience. 2024 May 14;546:20-32. doi: 10.1016/j.neuroscience.2024.03.020. Epub 2024 Mar 21. Neuroscience. 2024. PMID: 38521480 Review.
Cited by
- Cdk5 Modulates Long-Term Synaptic Plasticity and Motor Learning in Dorsolateral Striatum.
Hernandez A, Tan C, Mettlach G, Pozo K, Plattner F, Bibb JA. Hernandez A, et al. Sci Rep. 2016 Jul 22;6:29812. doi: 10.1038/srep29812. Sci Rep. 2016. PMID: 27443506 Free PMC article. - Reliance on habits at the expense of goal-directed control following dopamine precursor depletion.
de Wit S, Standing HR, Devito EE, Robinson OJ, Ridderinkhof KR, Robbins TW, Sahakian BJ. de Wit S, et al. Psychopharmacology (Berl). 2012 Jan;219(2):621-31. doi: 10.1007/s00213-011-2563-2. Epub 2011 Dec 3. Psychopharmacology (Berl). 2012. PMID: 22134475 Free PMC article. Clinical Trial. - Corticostriatal circuitry and habitual ethanol seeking.
Barker JM, Corbit LH, Robinson DL, Gremel CM, Gonzales RA, Chandler LJ. Barker JM, et al. Alcohol. 2015 Dec;49(8):817-24. doi: 10.1016/j.alcohol.2015.03.003. Epub 2015 May 14. Alcohol. 2015. PMID: 26059221 Free PMC article. Review. - Learning, Reward, and Decision Making.
O'Doherty JP, Cockburn J, Pauli WM. O'Doherty JP, et al. Annu Rev Psychol. 2017 Jan 3;68:73-100. doi: 10.1146/annurev-psych-010416-044216. Epub 2016 Sep 28. Annu Rev Psychol. 2017. PMID: 27687119 Free PMC article. Review. - Frequency-specific and D2 receptor-mediated inhibition of glutamate release by retrograde endocannabinoid signaling.
Yin HH, Lovinger DM. Yin HH, et al. Proc Natl Acad Sci U S A. 2006 May 23;103(21):8251-6. doi: 10.1073/pnas.0510797103. Epub 2006 May 12. Proc Natl Acad Sci U S A. 2006. PMID: 16698932 Free PMC article.
References
- Aberman JE, Salamone JD (1999) Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience 92: 545-552. - PubMed
- Adams CD (1982) Variation in the sensitivity of instrumental responding to reinforcer devaluation. Q J Exp Psychol B 34: 77-98.
- Adams CD, Dickinson A (1981) Actions and habits: variations in associative representations during instrumental learning. In: Information processing in animals: memory mechanisms (Spear NE, Miller RR, eds), pp 143-166. Hillsdale, NJ: Erlbaum.
- Adams S, Kesner RP, Ragozzino ME (2001) Role of the medial and lateral caudate-putamen in mediating an auditory conditional response association. Neurobiol Learn Mem 76: 106-116. - PubMed
- Amalric M, Moukhles H, Nieoullon A, Daszuta A (1995) Complex deficits on reaction time performance following bilateral intrastriatal 6-OHDA infusion in the rat. Eur J Neurosci 7: 972-980. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials