Human apolipoprotein E4 alters the amyloid-beta 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model - PubMed (original) (raw)
Comparative Study
Human apolipoprotein E4 alters the amyloid-beta 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model
John D Fryer et al. J Neurosci. 2005.
Abstract
Alzheimer's disease (AD) is characterized by the aggregation and deposition of the normally soluble amyloid-beta (Abeta) peptide in the extracellular spaces of the brain as parenchymal plaques and in the walls of cerebral vessels as cerebral amyloid angiopathy (CAA). CAA is a common cause of brain hemorrhage and is found in most patients with AD. As in AD, the epsilon4 allele of the apolipoprotein E (apoE) gene (APOE) is a risk factor for CAA. To determine the effect of human apoE on CAA in vivo, we bred human APOE3 and APOE4 "knock-in" mice to a transgenic mouse model (Tg2576) that develops amyloid plaques as well as CAA. The expression of both human apoE isoforms resulted in a delay in Abeta deposition of several months relative to murine apoE. Tg2576 mice expressing the more fibrillogenic murine apoE develop parenchymal amyloid plaques and CAA by 9 months of age. At 15 months of age, the expression of human apoE4 led to substantial CAA with very few parenchymal plaques, whereas the expression of human apoE3 resulted in almost no CAA or parenchymal plaques. Additionally, young apoE4-expressing mice had an elevated ratio of Abeta 40:42 in brain extracellular pools and a lower 40:42 ratio in CSF, suggesting that apoE4 results in altered clearance and transport of Abeta species within different brain compartments. These findings demonstrate that, once Abeta fibrillogenesis occurs, apoE4 favors the formation of CAA over parenchymal plaques and suggest that molecules or treatments that increase the ratio of Abeta 40:42 may favor the formation of CAA versus parenchymal plaques.
Figures
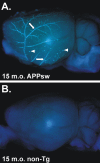
Figure 1.
Ex vivo image of an APPsw brain demonstrating extensive CAA. A, A 15-month-old (15 m.o.) APPsw mouse brain stained with X-34 and imaged with UV epifluorescence to denote fibrillar amyloid. Extensive CAA in leptomeningeal vessels can be seen on the cortical surface (arrows) as well as on plaques near the cortical surface (arrowheads). B, A 15-month-old non-transgenic (non-Tg) mouse brain stained with X-34, demonstrating the specificity of the stain.
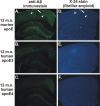
Figure 2.
Expression of human apoE3 or apoE4 delays deposition compared with murine apoE in the APPsw model at 12 months of age. A, D, Anti-Aβ immunostaining with monoclonal antibody m3D6 (A) or X-34 staining (D) to denote fibrillar amyloid of APPsw mice expressing endogenous murine apoE demonstrating CAA (arrows) and parenchymal plaques (arrowheads). However, 12-month-old (12 m.o.) APPsw mice expressing human apoE3 (B, E) or apoE4 (C, F) had little or no detectable plaques.
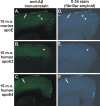
Figure 3.
Expression of apoE determines the level of CAA and parenchymal plaque pathology in an isoform-dependent manner in the 15-month-old (15 m.o.) APPsw model. A-F, Anti-Aβ immunostaining with m3D6 antibody (A-C) or X-34 staining (D-F) to visualize fibrillar Aβ deposits demonstrates CAA (arrows) and parenchymal plaques (arrowheads). A, D, APP swmice expressing endogenous murine apoE had both CAA and parenchymal plaques. B, C, E, F, APPsw mice expressing human apoE3 (B, E) had only occasional Aβ deposits, whereas APPsw mice expressing human apoE4 (C, F) had extensive CAA with infrequent parenchymal plaques.
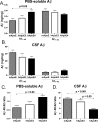
Figure 4.
Stereological quantitation of CAA and fibrillar plaque load. A-C, In 15-month-old APPsw mice, expression of human apoE4 (hApoE) and murine apoE (mApoE) resulted in significantly greater CAA load in leptomeningeal vessels (A), penetrating cortical vessels (B), and total cortical vessels (C) and parenchymal plaque load compared with mice expressing human apoE3. B-D, Expression of murine apoE also resulted in significantly higher levels of penetrating (B), total CAA (C), and parenchymal plaque load (D) compared with expression of human apoE4. D, Expression of both human apoE3 and apoE4 resulted in a dramatic reduction in parenchymal amyloid plaque load compared with mice expressing murine apoE. Error bars represent SEM.
Figure 5.
Fifteen-month-old APPsw mice expressing human apoE4 (hApoE4) had dramatically more deposition of Aβ incerebral vessels than mice expressing human apoE3. A, B, Human apoE3-expressing mice deposited 21.26 ± 5.097 ng/mg Aβ1-40 and 25.58 ± 9.282 ng/mg Aβ1-42 in cerebral vessels, whereas human apoE4-expressing mice deposited 21,040 ± 13,530 ng/mg Aβ1-40 and 4535 ± 2094 ng/mg Aβ1-42 in cerebral vessels. C, D, Additionally, human apoE3-expressing mice deposited 8.493 ± 1.918 ng/mg Aβ1-40 and 5.827 ± 1.421 ng/mg Aβ1-42 in brain parenchymal tissue, whereas human apoE4-expressing mice deposited 60.89 ± 20.53 ng/mg Aβ1-40 and 57.38 ± 17.48 ng/mg Aβ1-42 in brain parenchymal tissue. Error bars represent SEM.
Figure 6.
Expression of human apoE4 (hApoE4) alters the ratio of Aβ 40:42 in extracellular pools of the CNS. A, The level of Aβ1-40 in PBS-soluble brain extracts was significantly higher in APPsw mice expressing human apoE4 compared with mice expressing murine apoE (mApoE). Aβ1-42 levels were slightly, but not significantly, lower in APPsw mice expressing human apoE4 compared with mice expressing murine apoE. B, The level of Aβ1-40 in the CSF was slightly, but not significantly, lower in APPsw mice expressing human apoE4 compared with mice expressing murine apoE. Also, Aβ1-42 levels were slightly, but not significantly, higher in APPsw mice expressing human apoE4 compared with mice expressing murine apoE. C, The Aβ 40:42 ratio in PBS-soluble extracts was significantly higher in APPsw mice expressing human apoE4 compared with murine apoE. D, However, the Aβ 40:42 ratio in the CSF was significantly lower in APPsw mice expressing human apoE4 compared with mice expressing human apoE3 or murine apoE. Error bars represent SEM.
Figure 7.
Expression of murine apoE (mApoE) or human apoE3 (hApoE3) or apoE4 (hApoE4) does not alter apoE levels or total brain cholesterol. A, B, Levels of human apoE were not significantly different in PBS-soluble brain extracts of cortical tissue (A) or in CSF (B). C, Additionally, the level of hippocampal cholesterol did not significantly differ among mice expressing murine apoE, human apoE3, or human apoE4. Error bars represent SEM.
Similar articles
- Murine versus human apolipoprotein E4: differential facilitation of and co-localization in cerebral amyloid angiopathy and amyloid plaques in APP transgenic mouse models.
Liao F, Zhang TJ, Jiang H, Lefton KB, Robinson GO, Vassar R, Sullivan PM, Holtzman DM. Liao F, et al. Acta Neuropathol Commun. 2015 Nov 10;3:70. doi: 10.1186/s40478-015-0250-y. Acta Neuropathol Commun. 2015. PMID: 26556230 Free PMC article. - Apolipoprotein E markedly facilitates age-dependent cerebral amyloid angiopathy and spontaneous hemorrhage in amyloid precursor protein transgenic mice.
Fryer JD, Taylor JW, DeMattos RB, Bales KR, Paul SM, Parsadanian M, Holtzman DM. Fryer JD, et al. J Neurosci. 2003 Aug 27;23(21):7889-96. doi: 10.1523/JNEUROSCI.23-21-07889.2003. J Neurosci. 2003. PMID: 12944519 Free PMC article. - Modulation of Alzheimer-like synaptic and cholinergic deficits in transgenic mice by human apolipoprotein E depends on isoform, aging, and overexpression of amyloid beta peptides but not on plaque formation.
Buttini M, Yu GQ, Shockley K, Huang Y, Jones B, Masliah E, Mallory M, Yeo T, Longo FM, Mucke L. Buttini M, et al. J Neurosci. 2002 Dec 15;22(24):10539-48. doi: 10.1523/JNEUROSCI.22-24-10539.2002. J Neurosci. 2002. PMID: 12486146 Free PMC article. - Role of apoe/Abeta interactions in the pathogenesis of Alzheimer's disease and cerebral amyloid angiopathy.
Holtzman DM. Holtzman DM. J Mol Neurosci. 2001 Oct;17(2):147-55. doi: 10.1385/JMN:17:2:147. J Mol Neurosci. 2001. PMID: 11816788 Review. - The interaction of amyloid-beta with ApoE.
Carter DB. Carter DB. Subcell Biochem. 2005;38:255-72. doi: 10.1007/0-387-23226-5_13. Subcell Biochem. 2005. PMID: 15709483 Review.
Cited by
- APOE in the bullseye of neurodegenerative diseases: impact of the APOE genotype in Alzheimer's disease pathology and brain diseases.
Fernández-Calle R, Konings SC, Frontiñán-Rubio J, García-Revilla J, Camprubí-Ferrer L, Svensson M, Martinson I, Boza-Serrano A, Venero JL, Nielsen HM, Gouras GK, Deierborg T. Fernández-Calle R, et al. Mol Neurodegener. 2022 Sep 24;17(1):62. doi: 10.1186/s13024-022-00566-4. Mol Neurodegener. 2022. PMID: 36153580 Free PMC article. Review. - Mouse models of Alzheimer's disease.
Hall AM, Roberson ED. Hall AM, et al. Brain Res Bull. 2012 May 1;88(1):3-12. doi: 10.1016/j.brainresbull.2011.11.017. Epub 2011 Nov 28. Brain Res Bull. 2012. PMID: 22142973 Free PMC article. Review. - _APP_-Induced Patterned Neurodegeneration Is Exacerbated by APOE4 in Caenorhabditis elegans.
Sae-Lee W, Scott LL, Brose L, Encarnacion AJ, Shi T, Kore P, Oyibo LO, Ye C, Rozmiarek SK, Pierce JT. Sae-Lee W, et al. G3 (Bethesda). 2020 Aug 5;10(8):2851-2861. doi: 10.1534/g3.120.401486. G3 (Bethesda). 2020. PMID: 32580938 Free PMC article. - Blood-brain barrier leakage in Alzheimer's disease: From discovery to clinical relevance.
Nehra G, Bauer B, Hartz AMS. Nehra G, et al. Pharmacol Ther. 2022 Jun;234:108119. doi: 10.1016/j.pharmthera.2022.108119. Epub 2022 Jan 30. Pharmacol Ther. 2022. PMID: 35108575 Free PMC article. Review. - ApoE and Aβ in Alzheimer's disease: accidental encounters or partners?
Kanekiyo T, Xu H, Bu G. Kanekiyo T, et al. Neuron. 2014 Feb 19;81(4):740-54. doi: 10.1016/j.neuron.2014.01.045. Neuron. 2014. PMID: 24559670 Free PMC article. Review.
References
- Alonzo NC, Hyman BT, Rebeck GW, Greenberg SM (1998) Progression of cerebral amyloid angiopathy: accumulation of amyloid-beta40 in affected vessels. J Neuropathol Exp Neurol 57: 353-359. - PubMed
- Bales KR, Verina T, Dodel RC, Du Y, Altstiel L, Bender M, Hyslop P, Johnstone EM, Little SP, Cummins DJ, Piccardo P, Ghetti B, Paul SM (1997) Lack of apolipoprotein E dramatically reduces amyloid beta-peptide deposition. Nat Genet 17: 263-264. - PubMed
- Chalmers K, Wilcock GK, Love S (2003) APOE epsilon 4 influences the pathological phenotype of Alzheimer's disease by favouring cerebrovascular over parenchymal accumulation of A beta protein. Neuropathol Appl Neurobiol 29: 231-238. - PubMed
- Cirrito JR, May PC, O'Dell MA, Taylor JW, Parsadanian M, Cramer JW, Audia JE, Nissen JS, Bales KR, Paul SM, DeMattos RB, Holtzman DM (2003) In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-β metabolism and half-life. J Neurosci 23: 8844-8853. - PMC - PubMed
- Davis J, Xu F, Deane R, Romanov G, Previti ML, Zeigler K, Zlokovic BV, Van Nostrand WE (2004) Early-onset and robust cerebral microvascular accumulation of amyloid beta-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of amyloid beta-protein precursor. J Biol Chem 279: 20296-20306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG05681/AG/NIA NIH HHS/United States
- R01 NS034467/NS/NINDS NIH HHS/United States
- NS034467/NS/NINDS NIH HHS/United States
- R37 NS034467/NS/NINDS NIH HHS/United States
- AG13956/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- P01 AG011355/AG/NIA NIH HHS/United States
- R01 AG013956/AG/NIA NIH HHS/United States
- R37 AG013956/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous