Involvement of cannabinoid receptors in the regulation of neurotransmitter release in the rodent striatum: a combined immunochemical and pharmacological analysis - PubMed (original) (raw)
Comparative Study
Involvement of cannabinoid receptors in the regulation of neurotransmitter release in the rodent striatum: a combined immunochemical and pharmacological analysis
Attila Köfalvi et al. J Neurosci. 2005.
Abstract
Despite the profound effect of cannabinoids on motor function, and their therapeutic potential in Parkinson's and Huntington's diseases, the cellular and subcellular distributions of striatal CB1 receptors are not well defined. Here, we show that CB1 receptors are primarily located on GABAergic (vesicular GABA transporter-positive) and glutamatergic [vesicular glutamate transporter-1 (VGLUT-1)- and VGLUT-2-positive] striatal nerve terminals and are present in the presynaptic active zone, in the postsynaptic density, as well as in the extrasynaptic membrane. Both the nonselective agonist WIN552122 [(R)-(+)-[2,3-dihydro-5-methyl-3[(4-morpholinyl)methyl] pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl)methanone mesylate salt] (EC50, 32 nM) and the CB1-selective agonist ACEA [N-(2-chloroethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide] inhibited [3H]GABA release from rat striatal slices. The effect of these agonists was prevented by the CB1-selective antagonists SR141716A [N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide] (1 microM) and AM251 [1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-1-piperidinyl-1H-pyrazole-3-carboxamide trifluoroacetate salt] (1 microM), indicating that cannabinoids inhibit the release of GABA via activation of presynaptic CB1 receptors. Cannabinoids modulated glutamate release via both CB1 and non-CB1 mechanisms. Cannabinoid agonists and antagonists inhibited 25 mM K+-evoked [3H]glutamate release and sodium-dependent [3H]glutamate uptake. Partial involvement of CB1 receptors is suggested because low concentrations of SR141716A partly and AM251 fully prevented the effect of WIN552122 and CP55940 [5-(1,1-dimethylheptyl)-2-[5-hydroxy-2-(3-hydroxypropyl)cyclohexyl]phenol]. However, the effect of CB1 agonists and antagonists persisted in CB1 knock-out mice, indicating the involvement of non-CB1,CB1-like receptors. In contrast, cannabinoids did not modulate [3H]dopamine release or [3H]dopamine and [3H]GABA uptake. Our results indicate distinct modulation of striatal GABAergic and glutamatergic transmission by cannabinoids and will facilitate the understanding of the role and importance of the cannabinoid system in normal and pathological motor function.
Figures
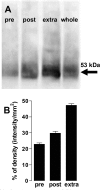
Figure 1.
CB1 receptors are located mainly extrasynaptically but also at the presynaptic active zone and at the postsynaptic density of rat striatal nerve terminals. A, Western blot (representative of 4 similar blots from different groups of animals) comparing CB1 receptor immunoreactivity, corresponding to the 53 kDa band, in a fraction enriched in the presynaptic active zone (pre), in the postsynaptic density (post), in the nerve terminals portion outside the active zone (extra), and in the initial synaptosomal fraction (whole), from which fractionation was performed. These fractions were obtained by pH fractionation, after solubilization of purified striatal nerve terminals as described in Materials and Methods. Thirty micrograms of protein of each fraction were applied into the SDS-PAGE gel, and a CB1 antibody was used at a 1:5000 dilution. B, Average distribution of CB1 receptor immunoreactivity in subsynaptic compartments. The CB1 receptor density was higher in the extrasynaptic fraction but was also present, to a lesser extent, in the presynaptic active zone and in the postsynaptic density.
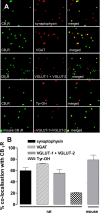
Figure 2.
CB1 receptors are present on GABAergic (from rat) and glutamatergic (from rat and mouse) and, to a lesser extent, on catecholaminergic (from rat) terminals. A, Representative double-labeling images of anti-CB1 receptor (CB1R) with anti-synaptophysin (marker of all nerve terminals), anti-VGAT (specific marker of GABAergic nerve terminals), anti-VGLUT-1 and anti-VGLUT-2 (specific markers of glutamatergic nerve terminals), and anti-Tyr-OH (specific marker of catecholaminergic nerve terminals). B, A summary of the extent of CB1 receptor colocalization with the specific markers of each type of nerve terminal (mean ± SEM of n = 4-6 plates) after counting ∼2000 terminals for each marker.
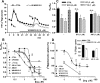
Figure 3.
Cannabinoid agonists inhibit the TTX-sensitive, electrically evoked release of [3H]GABA via CB1 receptor activation in rat striatal slices. A, [3H]GABA release from striatal slices in the control condition and in the presence of the nonselective cannabinoid agonist WIN55212-2. After 1 h of washout, 3 min samples were collected, as indicated by the _x_-axis, and counted for tritium. The sample tritium content was expressed as the percentage of the actual tissue tritium content at the time of the sample collection (FR%). The slices were stimulated twice with a pair of platinum electrodes (at 40 V, 2 Hz, 1 ms, 360 bipolar, square-wave pulses), as indicated by the arrows (electrical field stimulation, EFS1 and EFS2). The nonselective CB1 receptor agonist WIN55212-2 was applied, as indicated by the horizontal bar, 20 min before EFS2. WIN55212-2 decreased the second stimulation-evoked release of [3H]GABA. B, WIN55212-2 concentration dependently attenuated the evoked release of [3H]GABA but did not modify its uptake. ACEA, a highly selective CB1 receptor agonist, tested at 1 μ
m
, also significantly inhibited the release of [3H]GABA. C, The effect of WIN55212-2 (expressed with the EFS2/EFS1 ratio) is prevented by the selective CB1 receptor antagonists SR141716A (1 μ
m
) and AM251 (1 μ
m
), but not by coapplication of the AMPA/kainate receptor antagonist CNQX (10 μ
m
) and the NMDA receptor antagonist AP-5 (50 μ
m
). *p < 0.05; **p < 0.01. n ≥ 8 for all data points. CTRL, Control.
Figure 4.
Cannabinoids do not affect the TTX-sensitive, electrical field stimulation-evoked release and uptake of [3H]DA in rat striatal slices. A, B, Neither WIN55212-2 nor CP55940 modulated the basal outflow or evoked release of [3H]DA nor the uptake of [3H]DA under experimental conditions similar to those used for [3H]GABA (see Fig. 3 A, B). Note that WIN55212-2 also did not affect release if the frequency of stimulation was four times lower or five times higher (see Results). C, The lack of modulation by WIN55212-2 (expressed with the EFS2/EFS1 ratio) is not attributable to the presence of facilitatory or inhibitory polysynaptic mechanisms, because the blockade of ionotropic glutamate receptors by CNQX (10 μ
m
) and AP-5 (50 μ
m
) and by the GABAA antagonist bicuculline (20 μ
m
), respectively, did not reveal any WIN55212-2-mediated modulation, nor did the blockade of nitric oxide synthase by
l
-NAME (100 μ
m
). Activation of CB1 receptors by endogenous cannabinoids does not explain the lack of WIN55212-2 effect because the selective CB1 receptor antagonist SR141716A (10 μ
m
) applied 20 min before EFS2 had no effect on the second stimulation-evoked release of [3H]DA. n ≥ 6 for all data points. CTRL, Control.
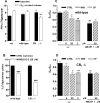
Figure 5.
Cannabinoids attenuate the Ca2+-dependent, 25 m
m
K+-evoked release and the temperature- and Na+-dependent uptake of [3H]glutamate in rat striatal synaptosomes. A, B, Arrows indicate the 3-min-long stimulation by high K+ superfusion (SK1 and SK2). The diagrams show that (in order of potency) CP55940, Δ9-THC, SR141716A, WIN55212-2, and AM251 (i.e., both agonists and antagonists of the CB1 receptor) concentration dependently inhibited the evoked release of [3H]glutamate. WIN55212-2 (6 μ
m
) and AM251 (30 μ
m
) displayed the greatest efficacy. WIN55212-3, the enantiomer inactive at the CB1 receptor, had no effect at 6 μ
m
. C, SR141716A (SR) attenuated by only one-third the effect of WIN55212-2 (WIN) or CP55940 (CP), but AM251 (AM) fully prevented the action of WIN55212-2 and CP55940. As expected, the evoked [3H]glutamate release was not sodium channel (i.e., TTX) dependent. D, WIN55212-2 inhibited the uptake of [3H]glutamate as well, which was prevented only by AM251. However, the effect of WIN55212-2 was nonstereoselective (i.e., non-CB1 receptor-mediated). AM251 more potently and effectively inhibited the uptake of [3H]glutamate than WIN55212-2. *p < 0.05; **p < 0.01. Only one point per ligand was marked for significance for the sake of simplicity. n ≥ 6 for all data points. CTRL, Control.
Figure 6.
Modulation of the uptake and release of [3H]glutamate in striatal synaptosomes of wild-type and CB1 receptor knock-out (-/-) mice. A, Properties of [3H]glutamate release in the rat and the two mouse types. The release is slightly but significantly altered in the CB1 -/- mouse compared with the wild-type mouse. B, WIN55212-2 inhibits the uptake of [3H]glutamate in both mouse types. C, D, WIN55212-2 (although less potently and effectively) and CP55940 attenuate the Ca2+-dependent, 25 m
m
K+-evoked release of [3H]glutamate from the wild-type CD-1 and the CB1 null-mutant mouse striatal synaptosomes. In the presence of AM251, given 20 min before SK1 and being present in all solutions used, the SK2/SK1 ratio significantly diminished, indicating differences between the CD-1 mouse and the Wistar rat, although the likely time-dependent underlying mechanism is unclear. No further inhibition of [3H]glutamate release by WIN55212-2 (20 μ
m
) is observed in the presence of AM251 either in wild-type or CB1-/-mice. *p < 0.05; **p < 0.01; ***p < 0.001 versus drug-freec ontrols (CTRL). n ≥ 8 for all data points.
Similar articles
- Cannabinoids inhibit the release of [3H]glutamate from rodent hippocampal synaptosomes via a novel CB1 receptor-independent action.
Köfalvi A, Vizi ES, Ledent C, Sperlágh B. Köfalvi A, et al. Eur J Neurosci. 2003 Oct;18(7):1973-8. doi: 10.1046/j.1460-9568.2003.02897.x. Eur J Neurosci. 2003. PMID: 14622229 - CB1-cannabinoid receptors are involved in the modulation of non-synaptic [3H]serotonin release from the rat hippocampus.
Balázsa T, Bíró J, Gullai N, Ledent C, Sperlágh B. Balázsa T, et al. Neurochem Int. 2008 Jan;52(1-2):95-102. doi: 10.1016/j.neuint.2007.07.008. Epub 2007 Jul 14. Neurochem Int. 2008. PMID: 17719142 - Blocking cannabinoid CB1 receptors for the treatment of nicotine dependence: insights from pre-clinical and clinical studies.
Le Foll B, Forget B, Aubin HJ, Goldberg SR. Le Foll B, et al. Addict Biol. 2008 Jun;13(2):239-52. doi: 10.1111/j.1369-1600.2008.00113.x. Addict Biol. 2008. PMID: 18482433 Free PMC article. Review. - 1-(2,4-Dichlorophenyl)-4-cyano-5-(4-[11C]methoxyphenyl)-_N_-(pyrrolidin-1-yl)-1_H_-pyrazole-3-carboxamide.
Leung K. Leung K. 2010 Jun 25 [updated 2010 Aug 26]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2010 Jun 25 [updated 2010 Aug 26]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20806446 Free Books & Documents. Review.
Cited by
- Endogenous content and release of [(3)H]-GABA and [(3)H]-glutamate in the spinal cord of chronically undernourished rat.
Quiróz-González S, Escartín-Pérez RE, Paz-Bermudez F, Segura-Alegría B, Reyes-Legorreta C, Guadarrama-Olmos JC, Florán-Garduño B, Jiménez-Estrada I. Quiróz-González S, et al. Neurochem Res. 2013 Jan;38(1):23-31. doi: 10.1007/s11064-012-0881-3. Epub 2012 Sep 15. Neurochem Res. 2013. PMID: 22983619 - G protein-coupled receptor heterocomplexes in neuropsychiatric disorders.
Moreno JL, Holloway T, González-Maeso J. Moreno JL, et al. Prog Mol Biol Transl Sci. 2013;117:187-205. doi: 10.1016/B978-0-12-386931-9.00008-8. Prog Mol Biol Transl Sci. 2013. PMID: 23663970 Free PMC article. Review. - The impact of phyto- and endo-cannabinoids on central nervous system diseases:A review.
Zhang SS, Zhang NN, Guo TT, Sheen LY, Ho CT, Bai NS. Zhang SS, et al. J Tradit Complement Med. 2022 Oct 12;13(1):30-38. doi: 10.1016/j.jtcme.2022.10.004. eCollection 2023 Jan. J Tradit Complement Med. 2022. PMID: 36685079 Free PMC article. Review. - Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum.
Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Uchigashima M, et al. J Neurosci. 2007 Apr 4;27(14):3663-76. doi: 10.1523/JNEUROSCI.0448-07.2007. J Neurosci. 2007. PMID: 17409230 Free PMC article. - Cannabinoid receptor signalling in neurodegenerative diseases: a potential role for membrane fluidity disturbance.
Maccarrone M, Bernardi G, Agrò AF, Centonze D. Maccarrone M, et al. Br J Pharmacol. 2011 Aug;163(7):1379-90. doi: 10.1111/j.1476-5381.2011.01277.x. Br J Pharmacol. 2011. PMID: 21323908 Free PMC article. Review.
References
- Bacci A, Huguenard JR, Prince DA (2004) Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature 431: 312-316. - PubMed
- Breivogel CS, Griffin G, Di Marzo V, Martin BR (2001) Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol 60: 155-163. - PubMed
- Brotchie JM (2003) CB1 cannabinoid receptor signaling in Parkinson's disease. Curr Opin Pharmacol 3: 54-61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases