Identification of a TcpC-TcpQ outer membrane complex involved in the biogenesis of the toxin-coregulated pilus of Vibrio cholerae - PubMed (original) (raw)
Identification of a TcpC-TcpQ outer membrane complex involved in the biogenesis of the toxin-coregulated pilus of Vibrio cholerae
Niranjan Bose et al. J Bacteriol. 2005 Apr.
Abstract
The toxin-coregulated pilus (TCP) of Vibrio cholerae and the soluble TcpF protein that is secreted via the TCP biogenesis apparatus are essential for intestinal colonization. The TCP biogenesis apparatus is composed of at least nine proteins but is largely uncharacterized. TcpC is an outer membrane lipoprotein required for TCP biogenesis that is a member of the secretin protein superfamily. In the present study, analysis of TcpC in a series of strains deficient in each of the TCP biogenesis proteins revealed that TcpC was absent specifically in a tcpQ mutant. TcpQ is a predicted periplasmic protein required for TCP biogenesis. Fractionation studies revealed that the protein is not localized to the periplasm but is associated predominantly with the outer membrane fraction. An analysis of the amount of TcpQ present in the series of tcp mutants demonstrated the inverse of the TcpC result (absence of TcpQ in a tcpC deletion strain). Complementation of the tcpQ deletion restored TcpC levels and TCP formation, and similarly, complementation of tcpC restored TcpQ. Metal affinity pull-down experiments performed using His-tagged TcpC or TcpQ demonstrated a direct interaction between TcpC and TcpQ. In the presence of TcpQ, TcpC was found to form a high-molecular-weight complex that is stable in 2% sodium dodecyl sulfate and at temperatures below 65 degrees C, a characteristic of secretin complexes. Fractionation studies in which TcpC was overexpressed in the absence of TcpQ showed that TcpQ is also required for proper localization of TcpC to the outer membrane.
Figures
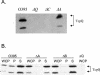
FIG. 1.
TcpC stability in tcp deletion strains. (A) Western immunoblot analysis of whole-cell lysates of tcp deletion mutants with TcpC antibody. (B) Periplasmic fractions of the tcp deletion strains. (C) Outer (OM) and inner (IM) membrane fractionations of the tcp deletion strains.
FIG. 2.
TcpQ stability and localization in wild-type and pilin and biogenesis mutant strains. (A) Whole-cell pellet lysates from wild-type, Δ_tcpQ_, Δ_tcpC_, and Δ_tcpA_ strains were electroblotted and probed with TcpQ antibody. (B) Fractionation profile of wild-type, Δ_tcpA_, and Δ_tcpB_ strains. WCP, whole-cell pellet lysates; P, periplasm; S, spheroplast. Arrows indicate the two forms of TcpQ.
FIG. 3.
TcpQ localization in wild-type and biogenesis mutant strains. (A) Inner and outer membrane fractions from wild-type cells probed with EpsL and TcpC (membrane controls) (B) Membrane fractionation profile of wild-type and biogenesis mutant strains. OM, outer membrane; IM, inner membrane. Arrows indicate the two forms of TcpQ.
FIG. 4.
TcpC and TcpQ localization patterns in tcpQ and tcpC deletion strains. (A) Immunoblot analysis of membrane and periplasmic fractions from wild-type (O395), Δ_tcpQ_ (pNB10), and O395 (pNB10) strains with anti-TcpC antibody. (B) Fractionation profile of TcpQ in Δ_tcpC_ (pNB11) and O395 (pNB11) strains with anti-TcpQ antibody. The first two lanes in both panels are wild-type and deletion strain controls. Per or per, periplasm; Sph, spheroplast; OM, outer membrane; IM, inner membrane.
FIG. 5.
TcpC and TcpQ interact directly as demonstrated by metal affinity chromatography. (A) Metal affinity chromatography performed with His-tagged TcpC. Fractions were separated by SDS-PAGE, and TcpQ was detected by immunoblot analysis. Elution fractions 1 and 2 (E1 and E2) were obtained using buffer containing 150 mM imidazole. Fractions 3 and 4 (E3 and E4) were obtained using buffer containing 100 mM EDTA in order to completely strip the beads. (B) The reciprocal experiment with metal affinity chromatography with His-tagged TcpQ and probing of the immunoblot with anti-TcpC antibody. FT, flowthrough.
FIG. 6.
TcpC forms a high-molecular-weight complex similar to that seen for other secretins. The whole-cell lysates from wild-type (WT), Δ_tcpC_, Δ_tcpQ_, Δ_tcpA_, and Δ_tcpS_ strains were resuspended in SDS-PAGE loading buffer and treated at 37 or 65°C or boiled for 5 min. Following SDS-PAGE, Western blot analysis with TcpC antibody was performed.
Similar articles
- Secretion of a soluble colonization factor by the TCP type 4 pilus biogenesis pathway in Vibrio cholerae.
Kirn TJ, Bose N, Taylor RK. Kirn TJ, et al. Mol Microbiol. 2003 Jul;49(1):81-92. doi: 10.1046/j.1365-2958.2003.03546.x. Mol Microbiol. 2003. PMID: 12823812 - Single amino acid substitutions in the N-terminus of Vibrio cholerae TcpA affect colonization, autoagglutination, and serum resistance.
Chiang SL, Taylor RK, Koomey M, Mekalanos JJ. Chiang SL, et al. Mol Microbiol. 1995 Sep;17(6):1133-42. doi: 10.1111/j.1365-2958.1995.mmi_17061133.x. Mol Microbiol. 1995. PMID: 8594332 - TCP pilus biosynthesis in Vibrio cholerae O1: gene sequence of tcpC encoding an outer membrane lipoprotein.
Ogierman MA, Manning PA. Ogierman MA, et al. FEMS Microbiol Lett. 1992 Oct 1;76(1-2):179-84. doi: 10.1016/0378-1097(92)90383-y. FEMS Microbiol Lett. 1992. PMID: 1358749 - Regulation of virulence in Vibrio cholerae.
Klose KE. Klose KE. Int J Med Microbiol. 2001 May;291(2):81-8. doi: 10.1078/1438-4221-00104. Int J Med Microbiol. 2001. PMID: 11437342 Review. - The toxin-co-regulated pilus of Vibrio cholerae O1: a model for type 4 pilus biogenesis?
Iredell JR, Manning PA. Iredell JR, et al. Trends Microbiol. 1994 Jun;2(6):187-92. doi: 10.1016/0966-842x(94)90109-i. Trends Microbiol. 1994. PMID: 7916248 Review.
Cited by
- Secretins: dynamic channels for protein transport across membranes.
Korotkov KV, Gonen T, Hol WG. Korotkov KV, et al. Trends Biochem Sci. 2011 Aug;36(8):433-43. doi: 10.1016/j.tibs.2011.04.002. Epub 2011 May 11. Trends Biochem Sci. 2011. PMID: 21565514 Free PMC article. Review. - Membrane association and multimerization of TcpT, the cognate ATPase ortholog of the Vibrio cholerae toxin-coregulated-pilus biogenesis apparatus.
Tripathi SA, Taylor RK. Tripathi SA, et al. J Bacteriol. 2007 Jun;189(12):4401-9. doi: 10.1128/JB.00008-07. Epub 2007 Apr 13. J Bacteriol. 2007. PMID: 17434972 Free PMC article. - Structure and function of PilQ, a secretin of the DNA transporter from the thermophilic bacterium Thermus thermophilus HB27.
Burkhardt J, Vonck J, Averhoff B. Burkhardt J, et al. J Biol Chem. 2011 Mar 25;286(12):9977-84. doi: 10.1074/jbc.M110.212688. Epub 2011 Feb 1. J Biol Chem. 2011. PMID: 21285351 Free PMC article. - Genetic mapping of secretion and functional determinants of the Vibrio cholerae TcpF colonization factor.
Krebs SJ, Kirn TJ, Taylor RK. Krebs SJ, et al. J Bacteriol. 2009 Jun;191(11):3665-76. doi: 10.1128/JB.01724-08. Epub 2009 Mar 20. J Bacteriol. 2009. PMID: 19304855 Free PMC article. - Not just an antibiotic target: Exploring the role of type I signal peptidase in bacterial virulence.
Walsh SI, Craney A, Romesberg FE. Walsh SI, et al. Bioorg Med Chem. 2016 Dec 15;24(24):6370-6378. doi: 10.1016/j.bmc.2016.09.048. Epub 2016 Sep 21. Bioorg Med Chem. 2016. PMID: 27769673 Free PMC article.
References
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology. Greene Publishing Associates and John Wiley & Sons, New York, N.Y.
- Bitter, W. 2003. Secretins of Pseudomonas aeruginosa: large holes in the outer membrane. Arch. Microbiol. 179:307-314. - PubMed
- Bitter, W., M. Koster, M. Latijnhouwers, H. de Cock, and J. Tommassen. 1998. Formation of oligomeric rings by XcpQ and PilQ, which are involved in protein transport across the outer membrane of Pseudomonas aeruginosa. Mol. Microbiol. 27:209-219. - PubMed
- Brok, R., P. Van Gelder, M. Winterhalter, U. Ziese, A. J. Koster, H. de Cock, M. Koster, J. Tommassen, and W. Bitter. 1999. The C-terminal domain of the Pseudomonas secretin XcpQ forms oligomeric rings with pore activity. J. Mol. Biol. 294:1169-1179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases