RhoE function is regulated by ROCK I-mediated phosphorylation - PubMed (original) (raw)
RhoE function is regulated by ROCK I-mediated phosphorylation
Kirsi Riento et al. EMBO J. 2005.
Abstract
The Rho GTPase family member RhoE regulates actin filaments partly by binding to and inhibiting ROCK I, a serine/threonine kinase that induces actomyosin contractility. Here, we show that ROCK I can phosphorylate multiple residues on RhoE in vitro. In cells, ROCK I-phosphorylated RhoE localizes in the cytosol, whereas unphosphorylated RhoE is primarily associated with membranes. Phosphorylation has no effect on RhoE binding to ROCK I, but instead increases RhoE protein stability. Using phospho-specific antibodies, we show that ROCK phosphorylates endogenous RhoE at serine 11 upon cell stimulation with platelet-derived growth factor, and that this phosphorylation requires an active protein kinase C signalling pathway. In addition, we demonstrate that phosphorylation of RhoE correlates with its activity in inducing stress fibre disruption and inhibiting Ras-induced transformation. This is the first demonstration of an endogenous Rho family member being phosphorylated in vivo and indicates that phosphorylation is an important mechanism to control the stability and function of this GTPase-deficient Rho protein.
Figures
Figure 1
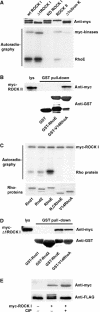
RhoE is phosphorylated by ROCK I. (A) Purified recombinant RhoE protein (2 μg) was incubated with the indicated myc-tagged kinases on beads in an in vitro kinase assay in the presence of [γ-32P]ATP. Proteins were resolved by SDS–PAGE and protein phosphorylation was detected by autoradiography. The presence of myc-tagged kinases was verified by immunoblotting. A fraction of wild-type (wt) ROCK I and kinase-dead (KD) ROCK I is C-terminally cleaved resulting in a smaller protein species (Coleman et al, 2001). (B) Lysates of COS7 cells expressing myc-ROCK II were incubated with the indicated GST-tagged proteins on beads, and the complexes were analysed by immunoblotting. (C) The constitutively active form of ROCK I, myc-Δ1ROCK I, on beads was incubated with 2 μg of each of the purified, recombinant Rho proteins in an in vitro kinase assay. Proteins were visualized by Coomassie staining, and protein phosphorylation was detected by autoradiography. N,CRhoE: His-ΔN,CRhoE (residues 16–200). (D) Lysates of COS7 cells expressing myc-Δ1ROCK I were incubated with GST-tagged proteins on beads and analysed by immunoblotting. (E) FLAG-RhoE was expressed in COS7 cells either alone or with myc-Δ1ROCK I, and immunoprecipitated with anti-FLAG antibody. For phosphatase treatment, the immunoprecipitates were incubated with calf intestinal phosphatase. FLAG-RhoE in the immunoprecipitates and the expression levels of myc-Δ1ROCK I in cell lysates were analysed by SDS–PAGE and immunoblotting.
Figure 2
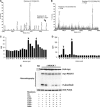
Identification of RhoE phosphorylation sites. (A, B) RhoE phosphorylated in vitro with myc-Δ1ROCK I was digested, the peptides were separated by reverse-phase chromatography, and the radioactive fractions were analysed by mass spectrometry. Masses of N-terminal (residues 2–16 plus six amino acids from GST (A)) and C-terminal (residues 216–235 or 215–235 (B)) singly phosphorylated peptides are shown. In both peptides, the methionine residue is oxidized. (C, D) Edman degradation of the radiolabelled N- and C-terminal RhoE peptides. The radioactive peaks of serines 7 and 11 (C) and serines 218 and 222 (D) are indicated by arrows. (E) Active myc-Δ1ROCK I or kinase-dead (KD) myc-ROCK I was incubated with wild-type or serine-to-alanine-mutated immunoprecipitated FLAG-RhoE in an in vitro kinase assay. Proteins were resolved by SDS–PAGE and protein phosphorylation was detected by autoradiography. The presence of myc-tagged kinases and FLAG-RhoE constructs was verified by immunoblotting.
Figure 3
Specificity of anti-pS7 and anti-pS11 antibodies. Wild-type or serine 7/11 to alanine-mutated FLAG-RhoE was expressed in COS7 cells either with or without myc-Δ1ROCK I. (A) The lysates were resolved by SDS–PAGE and immunoblotted with anti-FLAG and anti-myc antibodies. (B) FLAG-RhoE was immunoprecipitated with anti-FLAG antibody and detected by anti-pS7 or anti-pS11 antibodies. The protein expression was verified using anti-myc or anti-FLAG antibodies (cell lysates for ROCK and immunoprecipitates for RhoE). For ROCK inhibition, the cells were incubated with 10 μM Y-27632 for 12 h. For phosphatase treatment, the immunoprecipitates were incubated with protein phosphatase 1 (PP1) for 60 min, or under the same conditions without PP1 (Contr.).
Figure 4
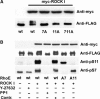
Phosphorylated RhoE localizes in the cytosol but phosphorylation is not required for the RhoE/ROCK I complex formation. (A) Wild-type (wt) GST-RhoE or a GST-RhoE construct in which all the seven ROCK I-phosphorylated residues were mutated to alanines (allA) were used for a pull-down assay of lysates of COS7 cells expressing active or kinase-dead (KD) myc-ROCK I (residues 1–727). After washes, the complexes were analysed directly (−) or after incubation under kinase assay conditions (ATP). The levels of GST-RhoE proteins and RhoE phosphorylation were elucidated by immunoblotting with anti-GST and anti-pS11 antibodies, respectively. The ROCK proteins associated with GST-RhoE constructs were detected by anti-myc antibody. (B) FLAG-tagged wild-type (wt) RhoE or allA-RhoE was expressed in COS7 cells together with myc-Δ1ROCK I. The cell membranes (M) were separated by membrane flotation, and analysed by immunoblotting with the phospho-specific RhoE antibodies. ERK1/2 and transferrin receptor are indicators of soluble and membrane fractions, respectively. Two nonspecific bands were often detected with anti-FLAG antibody (arrowhead). S: soluble fraction; T: postnuclear fraction.
Figure 5
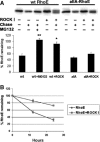
Phosphorylation increases RhoE protein stability. FLAG-tagged wild-type (wt) RhoE or allA-RhoE was expressed in COS7 cells with or without myc-Δ1ROCK I. Cellular proteins were metabolically labelled with 35S-Met, Cys, chased for 12 h (A, B) or 24 h (B), and FLAG-RhoE proteins were immunoprecipitated before and after the chase with anti-FLAG antibody. The immunoprecipitated proteins were resolved by SDS–PAGE and analysed by autoradiography. Bars represent percentage (±s.e.m., _n_=3) of FLAG-RhoE radioactivity remaining after the chase. The difference of wild-type (wt) RhoE expressed with ROCK I to that of RhoE expressed alone and the difference of RhoE in MG132-treated cells to that of nontreated cells are statistically significant (Student's _t_-test; *P<0.05, **P<0.01).
Figure 6
PDGF induces S11 phosphorylation on endogenous RhoE. Starved Swiss 3T3 cells were treated with PDGF (A–E) or LPA (C) for the indicated times, and after cell lysis, equal amounts of RhoE in cell lysates were analysed with anti-pS11 antibody. (A, D) Y-27632 or (B) H-1152 was added to the cells before PDGF stimulation to inhibit ROCK activity. Quantitation of S11 phosphorylation is shown in parentheses. (D) For MAPK inhibition, U0126, for PI3-kinase inhibition, LY294002, and (E) for PKC inhibition, GX109203X were added to the cells before cell stimulation. G: growing cells; S: starved cells.
Figure 7
PMA stimulation induces RhoE phosphorylation. (A) Starved Swiss 3T3 cells were treated with PDGF or PMA (20 ng/ml) for the indicated times. Equal amounts of total cell proteins were analysed by anti-pS11 and anti-RhoE antibodies. Activation of PKC was determined by detecting phosphorylation of the PKC target MARCKS. (B) GX109203X, a PKC inhibitor, or (C) Y-27632 or H-1152, ROCK inhibitors, were added to the cells before cell stimulation with 5 ng/ml PMA. (D) Starved HeLa cells were treated with 20 ng/ml PMA for 30 min and stained with phalloidin (red) to visualize filamentous (F-) actin, and with mouse anti-RhoE (blue) and rabbit anti-pS11 (green) antibodies. The bar represents 30 μm.
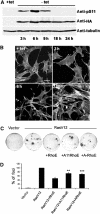
Figure 8
Phosphorylation of RhoE correlates with its activity. (A, B) RhoE-3T3 cells were grown in the presence (+tet) or absence (−tet) of tetracycline. HA-RhoE expression and S11 RhoE phosphorylation were analysed by immunoblotting using anti-HA and anti-pS11 antibodies, respectively (A). F-actin was detected by phalloidin staining (B). The bar represents 10 μm. (C, D) NIH 3T3 cells were transfected with the indicated expression plasmids and maintained in 5% serum for 12–15 days, replacing the medium every 2 days. After 12–15 days, cells were stained with crystal violet and the number of foci counted. (C) Plates from a single representative experiment, conducted in duplicate. (D) The mean values from three independent experiments as in (C), each conducted in duplicate, are shown in the graph, representing the percentage (±s.e.m.) of transformed foci relative to RasV12 transfectants. The differences in inhibition of Ras-induced transformation by the alanine-mutated RhoE forms (ARhoE: allA-RhoE) to that of wild-type RhoE are statistically significant (Student's _t_-test; **P<0.01, ***P<0.001).
Similar articles
- Inhibition of ROCK by RhoE.
Riento K, Ridley AJ. Riento K, et al. Methods Enzymol. 2006;406:533-41. doi: 10.1016/S0076-6879(06)06041-1. Methods Enzymol. 2006. PMID: 16472685 - Function and regulation of RhoE.
Riento K, Villalonga P, Garg R, Ridley A. Riento K, et al. Biochem Soc Trans. 2005 Aug;33(Pt 4):649-51. doi: 10.1042/BST0330649. Biochem Soc Trans. 2005. PMID: 16042565 Review. - N-terminus-mediated dimerization of ROCK-I is required for RhoE binding and actin reorganization.
Garg R, Riento K, Keep N, Morris JD, Ridley AJ. Garg R, et al. Biochem J. 2008 Apr 15;411(2):407-14. doi: 10.1042/BJ20071342. Biochem J. 2008. PMID: 18215121 - Rho and ROCK signaling in VEGF-induced microvascular endothelial hyperpermeability.
Sun H, Breslin JW, Zhu J, Yuan SY, Wu MH. Sun H, et al. Microcirculation. 2006 Apr-May;13(3):237-47. doi: 10.1080/10739680600556944. Microcirculation. 2006. PMID: 16627366 - Citron, a Rho target that affects contractility during cytokinesis.
Madaule P, Furuyashiki T, Eda M, Bito H, Ishizaki T, Narumiya S. Madaule P, et al. Microsc Res Tech. 2000 Apr 15;49(2):123-6. doi: 10.1002/(SICI)1097-0029(20000415)49:2<123::AID-JEMT3>3.0.CO;2-R. Microsc Res Tech. 2000. PMID: 10816250 Review.
Cited by
- Rnd3 coordinates early steps of cortical neurogenesis through actin-dependent and -independent mechanisms.
Pacary E, Azzarelli R, Guillemot F. Pacary E, et al. Nat Commun. 2013;4:1635. doi: 10.1038/ncomms2614. Nat Commun. 2013. PMID: 23535656 Free PMC article. - Serine-71 phosphorylation of Rac1 modulates downstream signaling.
Schwarz J, Proff J, Hävemeier A, Ladwein M, Rottner K, Barlag B, Pich A, Tatge H, Just I, Gerhard R. Schwarz J, et al. PLoS One. 2012;7(9):e44358. doi: 10.1371/journal.pone.0044358. Epub 2012 Sep 10. PLoS One. 2012. PMID: 22970203 Free PMC article. - Rnd3-induced cell rounding requires interaction with Plexin-B2.
McColl B, Garg R, Riou P, Riento K, Ridley AJ. McColl B, et al. J Cell Sci. 2016 Nov 1;129(21):4046-4056. doi: 10.1242/jcs.192211. Epub 2016 Sep 21. J Cell Sci. 2016. PMID: 27656111 Free PMC article. - Reduced expression of the ROCK inhibitor Rnd3 is associated with increased invasiveness and metastatic potential in mesenchymal tumor cells.
Belgiovine C, Frapolli R, Bonezzi K, Chiodi I, Favero F, Mello-Grand M, Dei Tos AP, Giulotto E, Taraboletti G, D'Incalci M, Mondello C. Belgiovine C, et al. PLoS One. 2010 Nov 30;5(11):e14154. doi: 10.1371/journal.pone.0014154. PLoS One. 2010. PMID: 21209796 Free PMC article. - The RHO Family GTPases: Mechanisms of Regulation and Signaling.
Mosaddeghzadeh N, Ahmadian MR. Mosaddeghzadeh N, et al. Cells. 2021 Jul 20;10(7):1831. doi: 10.3390/cells10071831. Cells. 2021. PMID: 34359999 Free PMC article. Review.
References
- Ballester R, Furth ME, Rosen OM (1987) Phorbol ester- and protein kinase C-mediated phosphorylation of the cellular Kirsten ras gene product. J Biol Chem 262: 2688–2695 - PubMed
- Barandier C, Ming XF, Rusconi S, Yang Z (2003) PKC is required for activation of ROCK by RhoA in human endothelial cells. Biochem Biophys Res Commun 304: 714–719 - PubMed
- Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF (2001) Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol 3: 339–345 - PubMed
- Ding J, Soule G, Overmeyer JH, Maltese WA (2003) Tyrosine phosphorylation of the Rab24 GTPase in cultured mammalian cells. Biochem Biophys Res Commun 312: 670–675 - PubMed
- Ellerbroek SM, Wennerberg K, Burridge K (2003) Serine phosphorylation negatively regulates RhoA in vivo. J Biol Chem 278: 19023–19031 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases