Genetic dissection and prognostic modeling of overt stroke in sickle cell anemia - PubMed (original) (raw)
Genetic dissection and prognostic modeling of overt stroke in sickle cell anemia
Paola Sebastiani et al. Nat Genet. 2005 Apr.
Abstract
Sickle cell anemia (SCA) is a paradigmatic single gene disorder caused by homozygosity with respect to a unique mutation at the beta-globin locus. SCA is phenotypically complex, with different clinical courses ranging from early childhood mortality to a virtually unrecognized condition. Overt stroke is a severe complication affecting 6-8% of individuals with SCA. Modifier genes might interact to determine the susceptibility to stroke, but such genes have not yet been identified. Using Bayesian networks, we analyzed 108 SNPs in 39 candidate genes in 1,398 individuals with SCA. We found that 31 SNPs in 12 genes interact with fetal hemoglobin to modulate the risk of stroke. This network of interactions includes three genes in the TGF-beta pathway and SELP, which is associated with stroke in the general population. We validated this model in a different population by predicting the occurrence of stroke in 114 individuals with 98.2% accuracy.
Conflict of interest statement
COMPETING INTERESTS STATEMENT
The authors declare competing financial interests (see the Nature Genetics website for details).
Figures
Figure 1
Examples of Bayesian network structures. (a) A simple Bayesian network with two nodes representing a SNP (G) and a phenotype (P). The probability distribution of G represents the genotype distribution in the population, and the conditional probability distribution of P describes the distribution of the phenotype given each genotype. (b) The association between G and P can be reversed using Bayes theorem. (c) A Bayesian network linking four SNPs (G1–G4) to a phenotype P. The phenotype is independent of the other SNPs, once we know the SNPs G3 and G4. The joint probability distribution of the network is fully specified by the five distributions representing the distribution of G1 (two parameters), of G2 given G1 (six parameters), of G3 given G2 (six parameters), of G4 given G2 (six parameters) and of P given G3 and G4 (nine parameters). The full probability distribution requires 81 × 2 − 1 = 161 parameters; this network requires only 29.
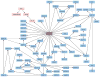
Figure 2
The Bayesian network describing the joint association of 69 SNPs with stroke. Nodes represent SNPs or clinical factors; the numbers after each gene distinguish different SNPs on the same gene. SNP are shown as blue nodes; their rs numbers are given in Supplementary Table 4 online. Clinical variables (HbF.G, fetal hemoglobin (g dL−1); HbF.P, fetal hemoglobin (%); HbG, total hemoglobin concentration; Thalassemia, heterozygosity or homozygosity with respect to a 3.7-kb α-thalassemia deletion) are shown as pink nodes. Twenty-five SNPs in ADCY9, ANXA2, BMP6, CCL2, CSF2, ECE1, ERG, MET, SELP, TEK and TGFBR3 are directly associated with the phenotype and have the largest independent effect on the risk of stroke. Note the association of stroke with several SNPs in ADCY9, BMP6, MET, SELP and TGFBR3, which usually reduces the possibility of false positives.
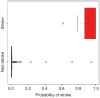
Figure 3
Box plot of the predictive probability of stroke (risk in 5 years) in an independent set of 7 individuals with stroke and 107 individuals without stroke. The plot shows a split of the predictive probabilities between these two outcomes: the predictive probabilities of stroke in the 7 individuals with stroke are >0.6, whereas the predictive probabilities of stroke in the 107 individuals without stroke are close to 0 (for only 2 individuals is the probability >0.5). The predictive probabilities are given in Supplementary Table 5 online.
Comment in
- Defining stroke risks in sickle cell anemia.
Meschia JF, Pankratz VS. Meschia JF, et al. Nat Genet. 2005 Apr;37(4):340-1. doi: 10.1038/ng0405-340. Nat Genet. 2005. PMID: 15800645 No abstract available.
Similar articles
- Defining stroke risks in sickle cell anemia.
Meschia JF, Pankratz VS. Meschia JF, et al. Nat Genet. 2005 Apr;37(4):340-1. doi: 10.1038/ng0405-340. Nat Genet. 2005. PMID: 15800645 No abstract available. - Gene interactions and stroke risk in children with sickle cell anemia.
Hoppe C, Klitz W, Cheng S, Apple R, Steiner L, Robles L, Girard T, Vichinsky E, Styles L; CSSCD Investigators. Hoppe C, et al. Blood. 2004 Mar 15;103(6):2391-6. doi: 10.1182/blood-2003-09-3015. Epub 2003 Nov 13. Blood. 2004. PMID: 14615367 - Genetic predictors for stroke in children with sickle cell anemia.
Flanagan JM, Frohlich DM, Howard TA, Schultz WH, Driscoll C, Nagasubramanian R, Mortier NA, Kimble AC, Aygun B, Adams RJ, Helms RW, Ware RE. Flanagan JM, et al. Blood. 2011 Jun 16;117(24):6681-4. doi: 10.1182/blood-2011-01-332205. Epub 2011 Apr 22. Blood. 2011. PMID: 21515823 Free PMC article. - Sickle hemoglobin (HbS) allele and sickle cell disease: a HuGE review.
Ashley-Koch A, Yang Q, Olney RS. Ashley-Koch A, et al. Am J Epidemiol. 2000 May 1;151(9):839-45. doi: 10.1093/oxfordjournals.aje.a010288. Am J Epidemiol. 2000. PMID: 10791557 Review. - Genetic etiologies for phenotypic diversity in sickle cell anemia.
Steinberg MH. Steinberg MH. ScientificWorldJournal. 2009 Jan 18;9:46-67. doi: 10.1100/tsw.2009.10. ScientificWorldJournal. 2009. PMID: 19151898 Free PMC article. Review.
Cited by
- Learning Bayesian Networks from Correlated Data.
Bae H, Monti S, Montano M, Steinberg MH, Perls TT, Sebastiani P. Bae H, et al. Sci Rep. 2016 May 5;6:25156. doi: 10.1038/srep25156. Sci Rep. 2016. PMID: 27146517 Free PMC article. - Genetics of stroke.
Guo JM, Liu AJ, Su DF. Guo JM, et al. Acta Pharmacol Sin. 2010 Sep;31(9):1055-64. doi: 10.1038/aps.2010.141. Epub 2010 Aug 23. Acta Pharmacol Sin. 2010. PMID: 20729874 Free PMC article. Review. - GenePING: secure, scalable management of personal genomic data.
Adida B, Kohane IS. Adida B, et al. BMC Genomics. 2006 Apr 26;7:93. doi: 10.1186/1471-2164-7-93. BMC Genomics. 2006. PMID: 16638151 Free PMC article. - My sister's keeper?: genomic research and the identifiability of siblings.
Cassa CA, Schmidt B, Kohane IS, Mandl KD. Cassa CA, et al. BMC Med Genomics. 2008 Jul 25;1:32. doi: 10.1186/1755-8794-1-32. BMC Med Genomics. 2008. PMID: 18655711 Free PMC article. - Interleukin-10 haplotypes are not associated with acute cerebral ischemia or high-risk transcranial Doppler in a newborn cohort of 395 children with sickle cell anemia.
Belisário AR, Sales RR, Toledo NE, Velloso-Rodrigues C, Silva CM, Viana MB. Belisário AR, et al. Rev Bras Hematol Hemoter. 2017 Apr-Jun;39(2):108-114. doi: 10.1016/j.bjhh.2016.09.017. Epub 2017 Feb 21. Rev Bras Hematol Hemoter. 2017. PMID: 28577646 Free PMC article.
References
- Adams RJ, et al. Stroke and conversion to high risk in children screened with transcranial Doppler ultrasound during the STOP study. Blood. 2004;103:3689–3694. - PubMed
- Steinberg MH, Forget BG, Higgs DR, Nagel RL. Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management. Cambridge University Press; Cambridge: 2001.
- Ware RE, Zimmerman SA, Schultz WH. Hydroxyurea as an alternative to blood transfusions for the prevention of recurrent stroke in children with sickle cell disease. Blood. 1999;94:3022–3026. - PubMed
- Adams RJ, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. - PubMed
- Taylor JGt, et al. Variants in the VCAM1 gene and risk for symptomatic stroke in sickle cell disease. Blood. 2002;100:4303–4309. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous