The FOXO3a transcription factor regulates cardiac myocyte size downstream of AKT signaling - PubMed (original) (raw)
. 2005 May 27;280(21):20814-23.
doi: 10.1074/jbc.M500528200. Epub 2005 Mar 21.
Yasuhiro Izumiya, Henrike Maatz, Peter Razeghi, Ichiro Shiojima, Marco Sandri, Kaori Sato, Ling Zeng, Stephan Schiekofer, David Pimentel, Stewart Lecker, Heinrich Taegtmeyer, Alfred L Goldberg, Kenneth Walsh
Affiliations
- PMID: 15781459
- PMCID: PMC3632436
- DOI: 10.1074/jbc.M500528200
The FOXO3a transcription factor regulates cardiac myocyte size downstream of AKT signaling
Carsten Skurk et al. J Biol Chem. 2005.
Abstract
Although signaling mechanisms inducing cardiac hypertrophy have been extensively studied, little is known about the mechanisms that reverse cardiac hypertrophy. Here, we describe the existence of a similar Akt/forkhead signaling axis in cardiac myocytes in vitro and in vivo, which is regulated by insulin, insulin-like growth factor (IGF), stretch, pressure overload, and angiotensin II stimulation. FOXO3a gene transfer prevented both IGF and stretch-induced hypertrophy in rat neonatal cardiac myocyte cultures in vitro. Transduction with FOXO3a also caused a significant reduction in cardiomyocyte size in mouse hearts in vivo. Akt/FOXO signaling regulated the expression of multiple atrophy-related genes "atrogenes," including the ubiquitin ligase atrogin-1 (MAFbx). In cardiac myocyte cultures, transduction with constitutively active Akt or treatment with IGF suppressed atrogin-1 mRNA expression, whereas transduction with FOXO3a stimulated its expression. FOXO3a transduction activated the atrogin-1 promoter in both cultured myocytes and mouse heart. Thus, in cardiomyocytes, as in skeletal muscle, FOXO3a activates an atrogene transcriptional program, which retards or prevents hypertrophy and is down-regulated by multiple physiological and pathological stimuli of myocyte growth.
Figures
Fig. 1. FOXO regulation in cardiac myocytes in vitro
Rat neonatal ventricular myocytes were cultured in the presence or absence of serum, the growth factors (IGF (A) and insulin (B)), or the PI 3-kinase inhibitor LY294002 (B). Levels of FOXO1, FOXO3a, FOXO4 protein and phosphorylation were determined with specific antibodies directed to total protein and the phosphorylation sites on serine 256 (FOXO1), threonine 32 (FOXO3a), and serine 193 (FOXO4), respectively. The phosphorylation status of Akt was also assessed at serine 473. Western blot membranes were stripped and reprobed for tubulin to compare loading in each lane. The Western blots shown are representative of three or more independent experiments.
Fig. 2. FOXO3a is regulated by Akt signaling
Subcellular localization of adenovirus-encoded WT-FOXO3a and TM-FOXO3a proteins. Cells were transduced with FOXO3a or Akt (constitutively active, MyrAkt; dominant-negative, DnAkt) replication-defective adenoviruses overnight as indicated. Subcellular FOXO3a distribution was determined with anti-FKHRL-1 and a rhodamine-conjugated secondary antibody by immunofluorescence. Upper left panel shows GFP, lower left panel illustrates nuclear staining (DAPI), upper middle panel indicates FOXO3a staining (rhodamine), and lower middle panel illustrates merged images for FOXO3a and DAPI. Upper and lower right panels depict higher magnification images of cells from FOXO3a-stained or FOXO3a- and DAPI-merged images from the middle panels that are indicated by the white arrows.
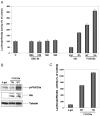
Fig. 3. Overexpression of FOXO3a promotes Akt phosphorylation
Rat neonatal ventricular myocytes were cultured in the presence or absence of serum. Cells were transduced with FOXO3a- or GFP-expressing replication-defective adenoviral vectors overnight. Levels of Akt protein and phosphorylation were determined with specific antibodies directed to total Akt1 protein and the phosphorylation at serine 473. Transgene expression was determined by anti-hemagglutinin antibody. Membranes were stripped and reprobed with tubulin as a loading control for each lane. This Western blot is representative of three independent experiments. TM, triple-mutant; HA, hemagglutinin.
Fig. 4. Growth factor-Akt-FOXO signaling axis in mouse hearts in vivo
Different transgenic mouse models were employed to study the regulation of FOXO3a in vivo. The mouse models were: CIRKO-insulin receptor knock-out mice; myrAkt-TG, transgenic mice with cardiac specific overexpression of Akt-1; Akt1−/−, Akt1 homozygous null mice. Also, wild-type mice were injected with insulin, 1 mg/kg body weight, and hearts were harvested after 10 min for analysis. Left ventricles were homogenized and cell lysates were used for Western blotting. Membranes were probed with anti-Akt1, anti-p-Akt (Ser473), anti-p-FOXO3a (Thr32), and anti-FOXO3a. Membranes were stripped and re-probed with tubulin as a loading control for each lane. Each Western blot is representative of three or more independent experiments. C, control (wild-type); iv, intravenously; TG, transgenic.
Fig. 5. Regulation of FOXO3a by pathological hypertrophic stimuli
NRVM were incubated with angiotensin II (AngII, 10−7 M) in vitro for 10 min. Transaortic constriction (TAC) was performed on C57BL/6 for 7 days. Lysates were used for Western blotting employing anti-Akt1 antibodies. Membranes were stripped and reprobed for _p-_Thr32-FOXO3a and total FOXO3a protein. Tubulin indicates equal loading for each lane. Each Western blot is representative of three or more independent experiments. C, control.
Fig. 6. FOXO3a inhibits growth factor-induced cardiac myocyte hypertrophy in vitro
NRVM were incubated in DMEM + 10% FBS on Lab-Tek® chamber slides in the presence or absence of IGF for 48 h. Parallel cultures of cells were also incubated in the absence of serum. After 24 h of IGF-1 incubation, cardiac myocytes were transduced with replication-defective adenoviral vectors encoding GFP (control), WT-FOXO3a, or TM-FOXO3a. Cardiomyocytes were identified by cardiac-specific staining for tropomyosin. Expression of the transgene was assessed by green fluorescent protein (the FOXO vectors also express GFP). Cell size was quantified 24 h following transduction using OpenLab® software. Data are presented as mean ± S.E. for 3 independent experiments (*, p < 0.05; **, p < 0.01 versus IGF).
Fig. 7. FOXO3a inhibits stretch-induced cardiac myocyte hypertrophy in vitro
NRVM were plated on Bioflex® culture plates, transduced with the indicated vectors, and stretched for 24 h as described under “Experimental Procedures.” Left panel, cell lysates were used for Western blotting to assess Ser473-Akt and Thr32-FOXO3a following stretch (S) and non-stretch (NS). Membranes were stripped and reprobed for tubulin as loading control. The Western blot is representative of three or more experiments. Right panel, bar graph indicating cardiomyocyte size. Cells were fixed and myocyte surface area was calculated employing NIH/OpenLab® software. Data are presented as mean ± S.E. for 3 independent experiments (*, p < 0.05; **, p < 0.01 versus stretch control).
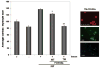
Fig. 8. In vivo FOXO3a gene transfer leads to reduction in cardiac cell size in mice
Adenoviral vectors expressing WT-FOXO3a or GFP were injected into the left ventricular wall of mice (109 pfu). A, 1 week after transduction, animals were sacrificed and expression of the transgene was determined by immunofluorescence staining for FOXO3a (left panels). Cell boundaries in the same sample were characterized by wheat germ agglutinin (WGA) staining (middle panels). Nuclear staining was employed using DAPI mounting medium. The right panels depict merged FOXO3a/WGA/DAPI images. Arrows indicate transgene positive cells. Lower panels indicate higher magnification of the cardiac myocytes depicted by white arrows. Transduced cells are a smaller size when compared with transgene-negative surrounding cardiac myocytes. B, quantification of cell size in both experimental groups. Data are presented as mean ± S.E. (**, p < 0.01). Transduced cells (n = 50) were compared with similar sections in the GFP group.
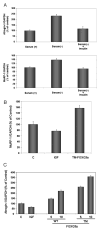
Fig. 9. FOXO3a induces expression of the ubiquitin ligases atrogin-1 and MuRF-1 in vivo
A, NRVM were cultured in DMEM + 10% FBS (Serum +) or DMEM + 0.5% FBS (Serum −) in the presence or absence of insulin. Quantitative RT-PCR for atrogin-1 and MuRF-1 was performed. Data are presented as mean ± S.E. B, FOXO3a regulates MuRF-1 expression in cardiac myocytes. C, FOXO3a regulates atrogin-1. QRT-PCR analysis in NRVM following adenoviral gene transfer of WT- or TM-FOXO3a. Parallel cultures were incubated with IGF-1 (50 ng/ml) overnight. Data are expressed as mean ± S.E. C, control; TM, triple-mutant.
Fig. 10. Atrogin-1 promoter activity is regulated by the Akt-FOXO signaling axis in vitro and in vivo
A, NRVM were transduced with the indicated adenoviral vectors and transfected with an atrogin-1-luciferase or Renilla reporter construct, respectively. Luciferase/Renilla activity was measured 12 h following transfection. Data are presented as mean ± S.E. for five independent experiments conducted in triplicate. B, adenoviral vectors expressing _β_-galactosidase (_β_-gal), WT-Ad-FOXO3a, or TM-FOXO3a (109 pfu each injection) were delivered to the left ventricular wall of mice together with 20 _μ_g of the atrogin-1 promoter plasmid. Hearts were snap-frozen 3 days following gene delivery and transgene expression was determined by Western blotting employing anti-hemagglutinin (HA) antibody. Membranes were stripped and reprobed for _p-_FOXO3a. Tubulin indicates the same loading in each lane. C, bar graph indicating activity of the luciferase construct in vivo. Data are presented as mean ± S.E. for three animals in each group.
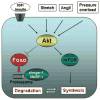
Fig. 11. Proposed scheme for Akt/FOXO-mediated regulation of cardiac myocyte size
Multiple inputs including growth factors, angiotensin II, stretch, and pressure overload lead to Akt phosphorylation. Akt induces hypertrophy in cardiac myocytes by increasing protein synthesis through the mammalian target of rapamycin-dependent pathways. Akt activation also leads to inactivation of forkhead transcription factors through phosphorylation. FOXO transcription factors activate atrogenes, including the ubiquitin ligases atrogin-1 and MuRF-1, which promote proteinolysis.
Similar articles
- Atrogin-1 inhibits Akt-dependent cardiac hypertrophy in mice via ubiquitin-dependent coactivation of Forkhead proteins.
Li HH, Willis MS, Lockyer P, Miller N, McDonough H, Glass DJ, Patterson C. Li HH, et al. J Clin Invest. 2007 Nov;117(11):3211-23. doi: 10.1172/JCI31757. J Clin Invest. 2007. PMID: 17965779 Free PMC article. - Adenosine monophosphate-activated protein kinase attenuates cardiomyocyte hypertrophy through regulation of FOXO3a/MAFbx signaling pathway.
Chen B, Wu Q, Xiong Z, Ma Y, Yu S, Chen D, Huang S, Dong Y. Chen B, et al. Acta Biochim Biophys Sin (Shanghai). 2016 Sep;48(9):827-32. doi: 10.1093/abbs/gmw076. Epub 2016 Aug 12. Acta Biochim Biophys Sin (Shanghai). 2016. PMID: 27521792 - IGF-1 prevents ANG II-induced skeletal muscle atrophy via Akt- and Foxo-dependent inhibition of the ubiquitin ligase atrogin-1 expression.
Yoshida T, Semprun-Prieto L, Sukhanov S, Delafontaine P. Yoshida T, et al. Am J Physiol Heart Circ Physiol. 2010 May;298(5):H1565-70. doi: 10.1152/ajpheart.00146.2010. Epub 2010 Mar 12. Am J Physiol Heart Circ Physiol. 2010. PMID: 20228261 Free PMC article. - FOXO3a mediates signaling crosstalk that coordinates ubiquitin and atrogin-1/MAFbx expression during glucocorticoid-induced skeletal muscle atrophy.
Zheng B, Ohkawa S, Li H, Roberts-Wilson TK, Price SR. Zheng B, et al. FASEB J. 2010 Aug;24(8):2660-9. doi: 10.1096/fj.09-151480. Epub 2010 Apr 6. FASEB J. 2010. PMID: 20371624 Free PMC article. - PI3 kinase regulation of skeletal muscle hypertrophy and atrophy.
Glass DJ. Glass DJ. Curr Top Microbiol Immunol. 2010;346:267-78. doi: 10.1007/82_2010_78. Curr Top Microbiol Immunol. 2010. PMID: 20593312 Review.
Cited by
- BMP type I receptor ALK2 is required for angiotensin II-induced cardiac hypertrophy.
Shahid M, Spagnolli E, Ernande L, Thoonen R, Kolodziej SA, Leyton PA, Cheng J, Tainsh RE, Mayeur C, Rhee DK, Wu MX, Scherrer-Crosbie M, Buys ES, Zapol WM, Bloch KD, Bloch DB. Shahid M, et al. Am J Physiol Heart Circ Physiol. 2016 Apr 15;310(8):H984-94. doi: 10.1152/ajpheart.00879.2015. Epub 2016 Feb 12. Am J Physiol Heart Circ Physiol. 2016. PMID: 26873969 Free PMC article. - miR-182 Modulates Myocardial Hypertrophic Response Induced by Angiogenesis in Heart.
Li N, Hwangbo C, Jaba IM, Zhang J, Papangeli I, Han J, Mikush N, Larrivée B, Eichmann A, Chun HJ, Young LH, Tirziu D. Li N, et al. Sci Rep. 2016 Feb 18;6:21228. doi: 10.1038/srep21228. Sci Rep. 2016. PMID: 26888314 Free PMC article. - Signaling Pathways That Control Muscle Mass.
Vainshtein A, Sandri M. Vainshtein A, et al. Int J Mol Sci. 2020 Jul 4;21(13):4759. doi: 10.3390/ijms21134759. Int J Mol Sci. 2020. PMID: 32635462 Free PMC article. Review. - Central Actions of Leptin Induce an Atrophic Pattern and Improves Heart Function in Lean Normoleptinemic Rats via PPARβ/δ Activation.
Rubio B, Pintado C, Mazuecos L, Benito M, Andrés A, Gallardo N. Rubio B, et al. Biomolecules. 2024 Aug 18;14(8):1028. doi: 10.3390/biom14081028. Biomolecules. 2024. PMID: 39199415 Free PMC article. - Mitochondrial Sirtuin 3: New emerging biological function and therapeutic target.
Zhang J, Xiang H, Liu J, Chen Y, He RR, Liu B. Zhang J, et al. Theranostics. 2020 Jul 9;10(18):8315-8342. doi: 10.7150/thno.45922. eCollection 2020. Theranostics. 2020. PMID: 32724473 Free PMC article. Review.
References
- Hunter JJ, Chien KR. N Engl J Med. 1999;341:1276–1283. - PubMed
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. N Engl J Med. 1990;322:1561–1566. - PubMed
- Sugden PH. Circ Res. 2003;93:1179–1192. - PubMed
- Pham FH, Cole SM, Clerk A. Adv Enzyme Regul. 2001;41:73– 86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL66957/HL/NHLBI NIH HHS/United States
- P01 HL066957/HL/NHLBI NIH HHS/United States
- R01 AG017241/AG/NIA NIH HHS/United States
- AR40197/AR/NIAMS NIH HHS/United States
- DK062307/DK/NIDDK NIH HHS/United States
- R01 AG015052/AG/NIA NIH HHS/United States
- AG15052/AG/NIA NIH HHS/United States
- R37 AG015052/AG/NIA NIH HHS/United States
- R01 AR040197/AR/NIAMS NIH HHS/United States
- R01 DK062307/DK/NIDDK NIH HHS/United States
- AG17241/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials