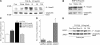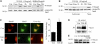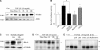TGF-{beta}1 activates two distinct type I receptors in neurons: implications for neuronal NF-{kappa}B signaling - PubMed (original) (raw)
TGF-{beta}1 activates two distinct type I receptors in neurons: implications for neuronal NF-{kappa}B signaling
Hans-Georg König et al. J Cell Biol. 2005.
Abstract
Transforming growth factor-betas (TGF-betas) are pleiotropic cytokines involved in development and maintenance of the nervous system. In several neural lesion paradigms, TGF-beta1 exerts potent neuroprotective effects. Neurons treated with TGF-beta1 activated the canonical TGF-beta receptor I/activin-like kinase receptor 5 (ALK5) pathway. The transcription factor nuclear factor-kappaB (NF-kappaB) plays a fundamental role in neuroprotection. Treatment with TGF-beta1 enhanced NF-kappaB activity in gelshift and reporter gene analyses. However, ectopic expression of a constitutively active ALK5 failed to mimic these effects. ALK1 has been described as an alternative TGF-beta receptor in endothelial cells. Interestingly, we detected significant basal expression of ALK1 and its injury-induced up-regulation in neurons. Treatment with TGF-beta1 also induced a pronounced increase in downstream Smad1 phosphorylation. Overexpression of a constitutively active ALK1 mimicked the effect of TGF-beta1 on NF-kappaB activation and neuroprotection. Our data suggest that TGF-beta1 simultaneously activates two distinct receptor pathways in neurons and that the ALK1 pathway mediates TGF-beta1-induced NF-kappaB survival signaling.
Figures
Figure 1.
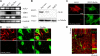
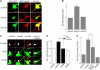
Neuronal cells express the alternative TGF-β type I receptor ALK1. (A) Expression of ALK5, TβRII, and ALK1 mRNA was determined by RT-PCR analysis in DIV14 primary cultures of rat hippocampal neurons, secondary cultures of astrocytes, as well as REF and PC12 cell lines. GAPDH expression is shown for normalization purposes. Reaction mixtures without reverse transcriptase served as controls for genomic DNA contamination in all cases (unpublished data). (B) Cell lysates of cultured rat hippocampal neurons, secondary astrocytes, and HepG2 cells were subjected to SDS-PAGE and Western blotting. The membranes were incubated with the ALK1 antibody, which recognized one specific band; α-tubulin band served as loading control. Experiments in duplicate yielded comparable results. (C) Immunofluorescence detection of ALK1 protein in cultured rat hippocampal neurons (red). DIV14 hippocampal cultures were fixed and subsequently incubated with ALK1 antibody. Preincubation of ALK1 antibody with blocking peptide and subsequent immunostaining served as negative control. βIII-Tubulin was used as a neuronal marker (green). (D and E) Immunofluorescence analysis of ALK1 protein expression in coronal sections of the adult rat brain. Representative cortical areas are shown. Slices were double-labeled with antibodies directed against ALK1 protein (red) and neuronal nuclear protein NeuN (D, green) or GFAP (E, green) and visualized by confocal microscopy (D) or confocal stack reconstruction (E). Red and green axes mark the positions of the corresponding perpendicular Z-layers. Bars, 10 μm.
Figure 2.
Injury-induced up-regulation of ALK1 receptor in vitro and in vivo. Evaluation of ALK1 mRNA (A) and protein levels (B) 24 h after a brief exposure to excitotoxic NMDA (300 μM; 5 min). (A) After the NMDA exposure, cells were treated with 10 ng/ml TGF-β1 or vehicle (2 mg/ml PBS/BSA). 30 cycles of PCR or Western blot for ALK1 receptor expression (B) was performed. GAPDH or actin served as control for equal loading. (C) Western blot analysis of ALK1 protein levels after ischemia/reoxygenation. Lysates were obtained from lesioned cortex and striatum of animals subjected to 1 h of ischemia followed by 12 or 24 h of reoxygenation (MCAO). (D) DAB-immunocytochemistry analysis of MCAO-induced cortical ALK1 expression. 24 h after reoxygenation, lesioned hemispheres were fixed and stained for ALK1 protein. Bar, 20 μm.
Figure 3.
TGF-β activates the ALK5 signaling pathway in cultured rat hippocampal neurons and astrocytes. (A) DIV14 rat hippocampal neurons were treated with 10 ng/ml TGF-β1. (B) In parallel experiments, cultures were incubated with 50 ng/ml BMP-6. Controls received vehicle. At the indicated time points, whole cell extracts were obtained in TOTEX buffer and analyzed after Western blotting with a P-Smad2 antibody. An unspecific band served as internal loading control as previously described (Goumans et al., 2002). A duplicate experiment yielded comparable results. (C and D) Dual-luciferase reporter gene analysis for the ALK5 pathway-responsive (CAGA)12-luc. 24 h after transfection of DIV6 hippocampal neurons, cells were treated with the indicated cytokines and lysed after 24 h of further incubation. In D, cotransfection was performed with the indicated plasmids. Luciferase responsiveness was normalized to the cotransfected RL-TK-luc. Data are means ± SEM from n = 4–6 cultures. *, P < 0.05 to control. Experiments were performed in duplicate with similar results. (E) Cultured secondary astrocytes were incubated with 10 ng/ml TGF-β1 as indicated. Subsequent procedures were conducted as specified in A and B.
Figure 4.
TGF-β1 induces ALK1 hetero-oligomerization with ALK5 and subsequent phosphorylation of Smad1. DIV14 hippocampal neurons (A) or secondary astrocytes (B) were incubated with the indicated cytokines, lysed, and subjected to SDS-PAGE and blotting (as described in Fig. 3 A). Membranes were incubated with P-Smad1 antibody. An unspecific band served as internal loading control as previously described (Goumans et al., 2002; compare B to Fig. 3 E, stripped membrane). The experiment was performed in triplicate with comparable results (A). (C) Immunofluorescence detection of Smad1 translocation to the nucleus and its quantitative evaluation in cultured rat hippocampal neurons. Neurons were treated with 10 ng/ml TGF-β1 for 3 h. After fixation and permeabilization, localization of Smad1 protein was determined by anti-Smad1 antibody (red). Neuronal nuclei were stained using NeuN antibody (green). Note the increase in Smad1 nuclear patches occurring in TGF-β1–treated cells, resembling neurons treated with BMP-4 (Angley et al., 2003). Bars, 10 μm. Quantification of nuclear translocation was determined without knowledge of the respective treatments. A total of 350–450 cells were counted in three separate experiments. Data are means ± SEM; *, P < 0.05. (D) Coimmunoprecipitation analysis of TGF-β1 induced ALK5/ALK1 receptor complexes. Cortical neurons (DIV14) were treated with10 ng/ml TGF-β1 for 15 min. After immunoprecipitation with ALK5 antibody, SDS-PAGE and Western blotting were conducted as described in Fig. 3 A. Membranes were incubated with the anti-ALK1 antibody. Immunodetection of light-chain (LC) by anti–rabbit antibody served as control. (E) Hippocampal neurons were incubated with 10 ng/ml TGF-β1 for 1 h. 30 min of preincubation with 5 μM SB-431542 were performed as indicated.
Figure 5.
TGF-β1 activates NF-κB in hippocampal neurons. (A) DIV14 cultured rat hippocampal neurons were treated with 10 ng/ml TGF-β1 as indicated. Whole cell lysates were subjected to SDS-PAGE and Western blotting. Serine-32 phosphorylated IκB-α was immunodetected by incubation of membranes with the polyclonal P-IκB-α antibody (top). For evaluation of overall IκB-α levels, membranes were stripped and reprobed with a rabbit polyclonal IκB-α antibody (bottom). (B) Dual-luciferase NF-κB reporter gene assay. After transfection of NF-κB-luc and RL-TK-luc, cells were allowed to recover for 24 h. 30 min of preincubation with 5 μM SB-431542 preceded 24 h of exposure to TGF-β1. 10 ng/ml TNF-α treatment served as positive control. Data are means ± SEM of n = 8–16 treatments in three independent, normalized, and pooled experiments. *, #, P < 0.05 compared with control or TGF-β1 treatment, respectively. (C) Western blot analysis for Bcl-xL protein content after 24 h of treatment with TGF-β1 in primary rat hippocampal neurons. Cells were preincubated for 30 min with 5 μM SB-431542 as indicated. After stripping, α-tubulin served as loading control. (D) Time course of enhanced κB-DNA binding after TGF-β1 treatment as revealed by gelshift experiments. Lysates were incubated with γ32P-ATP–labeled κB-consensus oligonucleotide (for comparative reasons, identical lysates as for the experiment depicted in Fig. 4 A were used). Experiments in triplicate revealed comparable results. Addition of IκB-α protein served as control for κB-binding specificity. The asterisk marks the NF-κB dimer-specific band. (E) Supershift analysis of TGF-β1–induced NF-κB complexes. After stimulation of hippocampal neurons with TGF-β1, equal amounts of lysates were analyzed by incubation with antibodies to the respective NF-κB subunits before incubation with labeled NF-κB oligonucleotide as denoted. Supershifted bands (ss) where visualized using autoradiography. A duplicate experiment yielded comparable results. (C and E) White lines indicate that intervening lanes have been spliced out.
Figure 6.
ALK1 mediates TGF-β–induced NF-κB activity, whereas overexpression of Smad6 abrogates this effect. (A) Primary rat hippocampal neurons were transfected with p65-EGFP and cotransfected with the constitutively active ALK encoding plasmids. 24 h after transfection, cells were fixed and stained with antiserum to neuronal marker βIII-tubulin (red). Localization of p65-EGFP (green) was evaluated using confocal microscopy as exemplified in the figure. In preliminary experiments, the amount of p65-EGFP was titrated down to a level still showing a homogeneous somatic distribution in a majority of neurons. Functionality of p65-EGFP was proven by its ability to increase NF-κB-luciferase reporter gene (unpublished data). Bars, 10 μm. (B) Dual-luciferase reporter gene assay for enhanced NF-κB activity after cotransfection of constitutively active TGF-β type I receptors. Hippocampal neurons were transfected on DIV6 with NF-κB-luciferase and RL-TK-luciferase reporter gene constructs, as well as cotransfected with either caALK1, caALK5, or pcDNA vector. 48 h after transfection, cells were lysed and dual-luciferase assay was performed. (C) Confocal immunofluorescence of increased nuclear accumulation of phosphorylated Smad1 in caALK1-transfected neurons. Hippocampal neurons were transiently cotransfected with pEGFP-N1 (green) and either control pcDNA, caALK1, or Smad6 expression vectors as indicated. 24 h after transfection, cells were fixed and immunostained with anti-P-Smad1 antibody (red). Arrowheads indicate cotransfected cells with increased versus decreased nuclear P-Smad1 staining. Bars, 10 μm. (D) Hippocampal neurons were transfected with the NF-κB-luciferase reporter plasmids and cotransfected with control or Smad6 vector after which they were allowed to recover from transfection for 24 h. Subsequent treatment with TGF-β1 was performed for a period of 24 h. (E) NF-κB-luciferase reporter gene transfected neurons were cotransfected with the indicated plasmids. 48 h after transfection, a dual-luciferase assay has been performed. Experiments performed in duplicate yielded similar results. The data represent NF-κB activity in relation to control ± SEM from n = 6 wells. *, P < 0.05 as compared with control or bracketed treatment.
Figure 7.
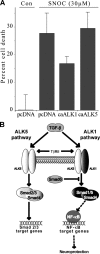
ALK1-initiated signaling confers neuroprotection. (A) Expression of the constitutively active ALK1 receptor results in increased resistance to oxidative stress. Cultured primary cortical neurons were cotransfected with caALK1, caALK5, or control vector together with EGFP. After transfer to antioxidant-depleted media, cultures were treated with 30 μM of the NO-donator SNOC. 24 h after treatment, cultures were incubated with PI for evaluation of cell death. 910–1226 EGFP-positive neurons were counted and depicted as means ± SEM of n = 3–6 treatments in two normalized and pooled experiments. (B) Proposed schematic model for TGF-β–induced downstream signaling in hippocampal neurons. TGF-β binds to TβRII, which recruits and activates the type I receptors. ALK1 and ALK5 phosphorylate their downstream targets Smad1/5 or Smad2/3, respectively. Moreover, Smad6 specifically inhibits the ALK1-induced pathway. Smad1/5-involving signaling is able to evoke NF-κB activation, whereas Smad2/3 induction results in activation of the classical TGF-β target genes.
Similar articles
- Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors.
Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Goumans MJ, et al. EMBO J. 2002 Apr 2;21(7):1743-53. doi: 10.1093/emboj/21.7.1743. EMBO J. 2002. PMID: 11927558 Free PMC article. - Role of Smad proteins in the regulation of NF-kappaB by TGF-beta in colon cancer cells.
Grau AM, Datta PK, Zi J, Halder SK, Beauchamp RD. Grau AM, et al. Cell Signal. 2006 Jul;18(7):1041-50. doi: 10.1016/j.cellsig.2005.08.021. Epub 2005 Nov 8. Cell Signal. 2006. PMID: 16288847 - ALK1 opposes ALK5/Smad3 signaling and expression of extracellular matrix components in human chondrocytes.
Finnson KW, Parker WL, ten Dijke P, Thorikay M, Philip A. Finnson KW, et al. J Bone Miner Res. 2008 Jun;23(6):896-906. doi: 10.1359/jbmr.080209. J Bone Miner Res. 2008. PMID: 18333754 - Transforming growth factor-beta: a neuroprotective factor in cerebral ischemia.
Dhandapani KM, Brann DW. Dhandapani KM, et al. Cell Biochem Biophys. 2003;39(1):13-22. doi: 10.1385/CBB:39:1:13. Cell Biochem Biophys. 2003. PMID: 12835526 Review. - Transforming growth factor beta signal transduction.
Dennler S, Goumans MJ, ten Dijke P. Dennler S, et al. J Leukoc Biol. 2002 May;71(5):731-40. J Leukoc Biol. 2002. PMID: 11994497 Review.
Cited by
- TGFβ signaling induces expression of Gadd45b in retinal ganglion cells.
Liu B, Sun X, Suyeoka G, Garcia JG, Leiderman YI. Liu B, et al. Invest Ophthalmol Vis Sci. 2013 Feb 5;54(2):1061-9. doi: 10.1167/iovs.12-10142. Invest Ophthalmol Vis Sci. 2013. PMID: 23329662 Free PMC article. - Activin A Promotes Osteoblastic Differentiation of Human Preosteoblasts through the ALK1-Smad1/5/9 Pathway.
Sugii H, Albougha MS, Adachi O, Tomita H, Tomokiyo A, Hamano S, Hasegawa D, Yoshida S, Itoyama T, Maeda H. Sugii H, et al. Int J Mol Sci. 2021 Dec 16;22(24):13491. doi: 10.3390/ijms222413491. Int J Mol Sci. 2021. PMID: 34948289 Free PMC article. - Neural Progenitor Cells Derived from Human Embryonic Stem Cells as an Origin of Dopaminergic Neurons.
Noisa P, Raivio T, Cui W. Noisa P, et al. Stem Cells Int. 2015;2015:647437. doi: 10.1155/2015/647437. Epub 2015 Apr 30. Stem Cells Int. 2015. PMID: 26064138 Free PMC article. - The neuroprotective functions of transforming growth factor beta proteins.
Dobolyi A, Vincze C, Pál G, Lovas G. Dobolyi A, et al. Int J Mol Sci. 2012;13(7):8219-8258. doi: 10.3390/ijms13078219. Epub 2012 Jul 3. Int J Mol Sci. 2012. PMID: 22942700 Free PMC article. - Role of transforming growth factor-beta in hematologic malignancies.
Dong M, Blobe GC. Dong M, et al. Blood. 2006 Jun 15;107(12):4589-96. doi: 10.1182/blood-2005-10-4169. Epub 2006 Feb 16. Blood. 2006. PMID: 16484590 Free PMC article. Review.
References
- Arsura, M., M. Wu, and G.E. Sonenshein. 1996. TGF beta 1 inhibits NF-kappa B/Rel activity inducing apoptosis of B cells: transcriptional activation of I kappa B alpha. Immunity. 5:31–40. - PubMed
- Attisano, L., J. Carcamo, F. Ventura, F.M. Weis, J. Massague, and J.L. Wrana. 1993. Identification of human activin and TGF beta type I receptors that form heteromeric kinase complexes with type II receptors. Cell. 75:671–680. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases