Targeting of Arf-1 to the early Golgi by membrin, an ER-Golgi SNARE - PubMed (original) (raw)
Targeting of Arf-1 to the early Golgi by membrin, an ER-Golgi SNARE
Akira Honda et al. J Cell Biol. 2005.
Abstract
Arf and Rab family GTPases regulate membrane traffic in cells, yet little is known about how they are targeted to distinct organelles. To identify sequences in Arf-1 necessary for Golgi targeting, we examined the localization of chimeras between Arf-1 and Arf-6. Here, we identify a 16-amino acid sequence in Arf-1 that specifies Golgi targeting and contains a motif (MXXE) that is important for Arf-1 binding to membrin, an ER-Golgi SNARE protein. The MXXE motif is conserved in all Arfs known to localize to the Golgi and enables Arf-1 to localize to the early Golgi. Arf-1 lacking these 16 aa can still localize to the late Golgi where it displays a more rapid Golgi-cytosol cycle than wild-type Arf-1. These studies suggest that membrin recruits Arf-1 to the early Golgi and reveal distinct kinetic cycles for Arf-1 at early and late Golgi determined by different sets of Arf regulators and effectors.
Figures
Figure 1.
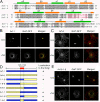
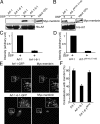
Subcellular localization of Arf chimeras. (A) Numbering and secondary structural elements (α helices and β sheets) of human Arf-1 and Arf-6 are shown. Identical residues between Arf-1 and Arf-6 are shaded in gray. Residues marked in red indicate amino acids 101–116 and 97–112 of Arf-1 and Arf-6, respectively. (B, C, and E) COS-7 cells overexpressing GalT-GFP and HA-tagged Arf-1 (B), untagged Arf-6 (C), or untagged Arf-6-1-6 (E) were untreated (Unt) or incubated in the presence of 20 μg/ml nocodazole for 2 h (+Nz). Cells were fixed and immunolabeled with antibodies against HA (B) or Arf-6 (C and E) followed by Alexa 594 anti–mouse and anti–rabbit antibodies, respectively. Bars, 10 μm. (D) Diagrams of chimeras of Arf-1 and Arf-6 are shown. Blue and yellow regions represent Arf-1 and Arf-6 sequences, respectively. + and − indicate ability to localize to the Golgi.
Figure 2.
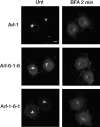
BFA-induced redistribution of Arf-6-1-6 and Arf-1-6-1. COS-7 cells overexpressing GFP-fused Arf-1, Arf-6-1-6, or Arf-1-6-1 were untreated (Unt) or incubated in the presence of 5 μg/ml BFA for 2 min and fixed. Bar, 10 μm.
Figure 3.
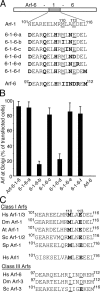
Identification of critical amino acids in residues 101–116 of Arf-1 for Golgi targeting of the Arf-6-1-6 chimera. (A) Sequence comparison of residues 101–116 of Arf-1 and corresponding residues of Arf-6 and mutants of Arf-6-1-6 are shown. Bold characters indicate residues unique to Arf-6. (B) COS-7 cells overexpressing GalT-GFP and the Arf constructs indicated were fixed and immunolabeled with antibodies against Arf-6 followed by Alexa 594 anti–rabbit antibodies. 100 cells were counted and the fraction of transfected cells that show clear colocalization of overexpressed Arf proteins with GalT-GFP was noted. The result shown is the mean ± SD for three experiments. (C) Sequence comparison of residues 101–116 of Arf-1 and corresponding residues of related proteins are shown. Bold characters indicate residues identical to 110M and 113E of Arf-1.
Figure 4.
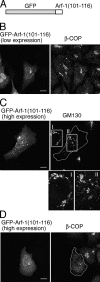
Amino acid residues 101–116 of Arf-1 are targeted to the Golgi. (A) Schematic representation of GFP-Arf-1(101–116). (B–D) HeLa cells expressing GFP-Arf-1(101–116) were fixed and immunolabeled with antibodies against β-COP (B and D) or GM130 (C) followed by Alexa 594 anti–rabbit and anti–mouse antibodies, respectively. Cells expressed GFP-Arf-1(101–116) at low to moderate level (B) or high level (C and D). The transfected cells in C and D are outlined. Images I and II in C show magnified views of boxed areas. Bars, 10 μm.
Figure 5.
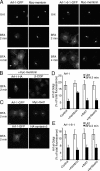
Arf-1, but not Arf-1-6-1, interacts and colocalizes with membrin. (A–D) His6-tagged Arf-1 and Arf-1-6-1, and Arf-1M110I, E113D were incubated with lysate from HeLa cells expressing myc-membrin in the presence of GDP. A cross-linking reagent DSP was added where indicated. Arfs were recovered and proteins were analyzed by immunoblotting. Shown is one experiment representative of three (A) or two (B) performed with similar results. In C and D, relative amount of membrin bound to His6-Arf for replicate experiments was determined using a densitometer. (E and F) HeLa (E) or NRK (F) cells overexpressing myc-membrin and Arf-1-GFP or Arf-1-6-1-GFP were fixed and immunolabeled with antibodies against myc followed by Alexa 594 anti–mouse antibodies. Bar, 10 μm. (F) Quantitative analysis of area overlap in the Golgi region for NRK cells as described in Materials and methods. Error bars are the mean ± SD.
Figure 6.
Membrin “protects” Arf-1 from BFA-induced redistribution. (A–C) COS-7 cells overexpressing myc-membrin and Arf-1-GFP or Arf-1-6-1-GFP (A), myc-membrin and Arf-1-HA (B), or Arf-1-GFP and myc-rbet1 or HA-syntaxin5 (C) were untreated (Unt) or incubated in the presence of 5 μg/ml BFA for indicated times and fixed. Cells were immunolabeled with antibodies against myc (A), HA and β-COP (B), or myc or HA (C) followed by Alexa 594 anti–mouse (A and C), or Alexa 488 anti–mouse and Alexa 594 anti–rabbit (B) antibodies. Bars, 10 μm. (D and E) HeLa cells overexpressing Arf-1-GFP alone (D, control) or Arf-1-6-1-GFP alone (E, control), or with myc-membrin or myc-rbet1, or HA-syntaxin5 were untreated or incubated in the presence of 5 μg/ml BFA for 2 min and fixed and processed for immunolabeling with antibodies against myc or HA followed by Alexa 594 anti–mouse and anti–rabbit antibodies, respectively. To quantify Golgi and non-Golgi pools of Arf-1-GFP, one region of interest was drawn around Golgi and the other region of interest was drawn around the rest of the cell (representing total cellular fluorescence). The mean fluorescence intensity associated with the Golgi and total cell was measured for Arf-1-GFP. The total Arf-1-GFP fluorescence associated with the Golgi was expressed as a fraction of the total cellular fluorescence, and this procedure was repeated for 10 cells. Error bars are the mean ± SD.
Figure 7.
Anterograde movement of membrin and Arf-1 into the Golgi in BFA-recovering cells. (A) NRK cells overexpressing myc-membrin and Arf-1-GFP or Arf-1-6-1-GFP were incubated in the presence of 5 μg/ml BFA for 30 min, washed free of BFA, and incubated in the absence of BFA for the indicated times of wash out (WO) and fixed. Cells were immunolabeled with antibodies against myc followed by Alexa 594 anti–mouse antibodies. (B) NRK cells overexpressing myc-membrin were treated as in A and fixed. Cells were immunolabeled with antibodies against myc and β-COP followed by Alexa 488 anti–mouse and Alexa 594 anti–rabbit antibodies. Bars, 10 μm.
Figure 8.
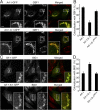
Comparison of the distribution of Arf-1 and Arf-1-6-1 to Arf-GEFs. NRK cells overexpressing GFP-fused Arf-1, Arf-1-6-1, and Arf-1-6-1MXXE were fixed and immunolabeled with antibodies against GBF1 (A) or BIG1 (C) followed by Alexa 594 anti–mouse and anti–rabbit antibodies, respectively. Bars, 10 μm. (B and D) Quantitative analysis of area overlap in the Golgi region as in A and C. Error bars are the mean ± SD.
Figure 9.
Arf-1 and Arf-1-6-1 are regulated differently at Golgi membranes. (A) The kinetics of recovery of GFP-fused Arf-1, Arf-1-6-1, and Arf-1-6-1MXXE in the Golgi region after photobleaching are shown. The Golgi regions of cells expressing Arfs-GFP were photobleached at maximal laser intensity. Immediately after the bleach, cells were scanned every 5 s. The fluorescence intensity in the bleach area was measured. Shown is one experiment representative of three performed with similar results. (B) Models for membrane association/dissociation cycle of Arf-1 and Arf-1-6-1. See text for details.
Similar articles
- Correct targeting of plant ARF GTPases relies on distinct protein domains.
Matheson LA, Suri SS, Hanton SL, Chatre L, Brandizzi F. Matheson LA, et al. Traffic. 2008 Jan;9(1):103-20. doi: 10.1111/j.1600-0854.2007.00671.x. Epub 2007 Nov 27. Traffic. 2008. PMID: 17988226 - Knockout of Arfrp1 leads to disruption of ARF-like1 (ARL1) targeting to the trans-Golgi in mouse embryos and HeLa cells.
Zahn C, Hommel A, Lu L, Hong W, Walther DJ, Florian S, Joost HG, Schürmann A. Zahn C, et al. Mol Membr Biol. 2006 Nov-Dec;23(6):475-85. doi: 10.1080/09687860600840100. Mol Membr Biol. 2006. PMID: 17127620 - A SNARE involved in protein transport through the Golgi apparatus.
Lowe SL, Peter F, Subramaniam VN, Wong SH, Hong W. Lowe SL, et al. Nature. 1997 Oct 23;389(6653):881-4. doi: 10.1038/39923. Nature. 1997. PMID: 9349823 - Localization and function of Arf family GTPases.
Donaldson JG, Honda A. Donaldson JG, et al. Biochem Soc Trans. 2005 Aug;33(Pt 4):639-42. doi: 10.1042/BST0330639. Biochem Soc Trans. 2005. PMID: 16042562 Review. - Membrane recruitment of effector proteins by Arf and Rab GTPases.
Kawasaki M, Nakayama K, Wakatsuki S. Kawasaki M, et al. Curr Opin Struct Biol. 2005 Dec;15(6):681-9. doi: 10.1016/j.sbi.2005.10.015. Epub 2005 Nov 9. Curr Opin Struct Biol. 2005. PMID: 16289847 Review.
Cited by
- Recruitment of Arf1-GDP to Golgi by Glo3p-type ArfGAPs is crucial for golgi maintenance and plant growth.
Min MK, Jang M, Lee M, Lee J, Song K, Lee Y, Choi KY, Robinson DG, Hwang I. Min MK, et al. Plant Physiol. 2013 Feb;161(2):676-91. doi: 10.1104/pp.112.209148. Epub 2012 Dec 24. Plant Physiol. 2013. PMID: 23266962 Free PMC article. - Interaction of SNAREs with ArfGAPs precedes recruitment of Sec18p/NSF.
Schindler C, Spang A. Schindler C, et al. Mol Biol Cell. 2007 Aug;18(8):2852-63. doi: 10.1091/mbc.e06-08-0756. Epub 2007 May 23. Mol Biol Cell. 2007. PMID: 17522384 Free PMC article. - BioID Performed on Golgi Enriched Fractions Identify C10orf76 as a GBF1 Binding Protein Essential for Golgi Maintenance and Secretion.
Chan CJ, Le R, Burns K, Ahmed K, Coyaud E, Laurent EMN, Raught B, Melançon P. Chan CJ, et al. Mol Cell Proteomics. 2019 Nov;18(11):2285-2297. doi: 10.1074/mcp.RA119.001645. Epub 2019 Sep 13. Mol Cell Proteomics. 2019. PMID: 31519766 Free PMC article. - Arf GTPases Are Required for the Establishment of the Pre-Assembly Compartment in the Early Phase of Cytomegalovirus Infection.
Pavišić V, Mahmutefendić Lučin H, Blagojević Zagorac G, Lučin P. Pavišić V, et al. Life (Basel). 2021 Aug 23;11(8):867. doi: 10.3390/life11080867. Life (Basel). 2021. PMID: 34440611 Free PMC article. - Retrograde traffic from the Golgi to the endoplasmic reticulum.
Spang A. Spang A. Cold Spring Harb Perspect Biol. 2013 Jun 1;5(6):a013391. doi: 10.1101/cshperspect.a013391. Cold Spring Harb Perspect Biol. 2013. PMID: 23732476 Free PMC article. Review.
References
- Allan, B.B., B.D. Moyer, and W.E. Balch. 2000. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science. 289:444–448. - PubMed
- Amor, J.C., D.H. Harrison, R.A. Kahn, and D. Ringe. 1994. Structure of the human ADP-ribosylation factor 1 complexed with GDP. Nature. 372:704–708. - PubMed
- Antonny, B., S. Beraud-Dufour, P. Chardin, and M. Chabre. 1997. N-terminal hydrophobic residues of the G-protein ADP-ribosylation factor-1 insert into membrane phospholipids upon GDP to GTP exchange. Biochemistry. 36:4675–4684. - PubMed
- Bonifacino, J.S., and B.S. Glick. 2004. The mechanisms of vesicle budding and fusion. Cell. 116:153–166. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous