Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain - PubMed (original) (raw)
Comparative Study
. 2005 Mar 23;25(12):3126-31.
doi: 10.1523/JNEUROSCI.3815-04.2005.
Heidi Röhrich, Theodore H Lindsay, Molly A Sevcik, Matthew J Schwei, Kazufumi Kubota, Kyle G Halvorson, Jeannie Poblete, Sandra R Chaplan, Adrienne E Dubin, Nicholas I Carruthers, Devin Swanson, Michael Kuskowski, Christopher M Flores, David Julius, Patrick W Mantyh
Affiliations
- PMID: 15788769
- PMCID: PMC6725088
- DOI: 10.1523/JNEUROSCI.3815-04.2005
Comparative Study
Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain
Joseph R Ghilardi et al. J Neurosci. 2005.
Abstract
Cancer colonization of bone leads to the activation of osteoclasts, thereby producing local tissue acidosis and bone resorption. This process may contribute to the generation of both ongoing and movement-evoked pain, resulting from the activation of sensory neurons that detect noxious stimuli (nociceptors). The capsaicin receptor TRPV1 (transient receptor potential vanilloid subtype 1) is a cation channel expressed by nociceptors that detects multiple pain-producing stimuli, including noxious heat and extracellular protons, raising the possibility that it is an important mediator of bone cancer pain via its capacity to detect osteoclast- and tumor-mediated tissue acidosis. Here, we show that TRPV1 is present on sensory neuron fibers that innervate the mouse femur and that, in an in vivo model of bone cancer pain, acute or chronic administration of a TRPV1 antagonist or disruption of the TRPV1 gene results in a significant attenuation of both ongoing and movement-evoked nocifensive behaviors. Administration of the antagonist had similar efficacy in reducing early, moderate, and severe pain-related responses, suggesting that TRPV1 may be a novel target for pharmacological treatment of chronic pain states associated with bone cancer metastasis.
Figures
Figure 3.
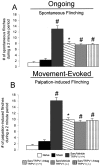
Administration of a TRPV1 antagonist reduces bone cancer-induced pain-related behaviors and retains its analgesic efficacy with disease progression. A, C, Tumor-induced ongoing nocifensive behaviors were evaluated by measuring spontaneous guarding (A) and spontaneous flinching (C) over a 2 min observation period. B, D, Movement-evoked allodynia was assessed by measuring the time spent guarding (B) and flinching (D) over a 2 min observation period after normally non-noxious palpation of the distal femur. A-D, Note that, in mice with bone cancer, there is a significant increase in the duration and magnitude of guarding (A, B) and flinching (C, D). Chronic treatment with the TRPV1 antagonist JNJ-17203212 (30 mg/kg, s.c.; twice daily), administered from 6 to 18 d after tumor injection significantly reduced parameters of both ongoing and movement-evoked pain-related behaviors compared with sarcoma (sarc)/vehicle (veh) animals. E, F, Note also that, at all time points examined (days 9-18), chronic administration of JNJ-17203212 (30 mg/kg, s.c.; twice daily) maintained significant analgesic efficacy with disease progression, during which the severity of pain-related behaviors increased. n > 8 for all experimental categories with the exception of E, F, in which n = 4 for the sarcoma/TRPV1 antagonist group. Error bars represent SEM. #p < 0.05, sham/vehicle versus sarcoma/vehicle; *p < 0.05, sarcoma/TRPV1 antagonist versus sarcoma/vehicle (A-D, one-way ANOVA; E, F, one-way ANOVA at each time point).
Figure 1.
A subpopulation of sensory nerve fibers that innervate the bone express the TRPV1 channel. Confocal photomicrographs showing TRPV1-expressing nerve fibers in the normal and tumor-bearing bone. A, In the marrow space of the normal bone, TRPV1-expressing nerve fibers are closely associated with blood vessels similar to observations in other peripheral vascular beds. B, After tumor invasion of the marrow space, TRPV1-expressing sensory fibers remain associated with blood vessels at the leading edge of the tumor, but these fibers are not as ramified as those found in the normal bone. C, As tumor growth continues, TRPV1-expressing fibers can still be found deep within the tumor, but, in general, the fibers begin to have a fragmented appearance, suggesting tumor-induced destruction of the distal processes of the sensory nerve fibers. NM, Normal marrow; T, tumor. B, Leading edge. C, Major body of tumor mass.
Figure 2.
Sensory nerve fibers that innervate the tumor-bearing mouse femur maintain their expression of TRPV1 with disease progression. A, B, A population of small- to medium-sized neurons in the contralateral DRG normally express the TRPV1 channel (A; red) and the percentage and size of sensory neurons expressing the TRPV1 in ipsilateral L2 DRG (B) that innervate the tumor-bearing femur do not change at 14 d after tumor injection (33.3 ± 1.7% contralateral vs 32.1 ± 4.3% ipsilateral; n = 4), when significant hyperalgesia has developed. Fourteen days after tumor injection, tumor cells have invaded the marrow space and mineralized bone, seem to injure the distal processes of sensory nerve fibers that innervate the bone, and, compared with the contralateral DRG (C), induced a significant upregulation of ATF-3 (blue) in sensory neurons of the ipsilateral DRG (D). Double-label immunohistochemistry, merging the images obtained in A and C (E) or in B and D (F), suggests that a population of TRPV1-expressing sensory neurons innervate the tumor-bearing bone and exhibit an injured phenotype, as demonstrated by ATF-3 coexpression. Moreover, both the percentage of cells expressing TRPV1 immunoreactivity and their level of TRPV1 expression were similar in DRG neurons innervating normal and tumor-bearing bone. Scale bar: (in F) A-F, 50 μm.
Figure 4.
Attenuation of bone cancer-induced pain-related behaviors and lack of additional analgesic effect of a TRPV1 antagonist (Antag) in TRPV1 null mice. A, B, Mice lacking a functional TRPV1 show a significant decrease in numbers of ongoing (A) and movement-evoked (B) pain-related behaviors compared with wild-type C3H/HeJ animals when behavioral testing is performed 10-14 d after tumor injection. Note that the reduction in pain-related behaviors in the TRPV1 null mice is approximately the same as that seen in the tumor-bearing wild-type C3H/HeJ animals treated with JNJ-17203212 (Fig. 3) and that TRPV1 null mice treated acutely with 30 mg/kg subcutaneously of JNJ-17203212 showed no additional reduction in pain-related behaviors, suggesting that this compound is exerting its action by antagonizing the TRPV1 channel. Data for naive and sham experimental categories were obtained using TRPV1+/+ bred mice. Error bars represent SEM. #p < 0.05, versus sham/vehicle; *p < 0.05, versus sarcoma (sarc)/TRPV1 +/+ (one-way ANOVA).
Similar articles
- Bone cancer increases transient receptor potential vanilloid subfamily 1 expression within distinct subpopulations of dorsal root ganglion neurons.
Niiyama Y, Kawamata T, Yamamoto J, Omote K, Namiki A. Niiyama Y, et al. Neuroscience. 2007 Aug 24;148(2):560-72. doi: 10.1016/j.neuroscience.2007.05.049. Epub 2007 Jul 25. Neuroscience. 2007. PMID: 17656027 - Involvement of TRPV1 in nociceptive behavior in a rat model of cancer pain.
Shinoda M, Ogino A, Ozaki N, Urano H, Hironaka K, Yasui M, Sugiura Y. Shinoda M, et al. J Pain. 2008 Aug;9(8):687-99. doi: 10.1016/j.jpain.2008.02.007. Epub 2008 May 2. J Pain. 2008. PMID: 18455478 - Repeated dosing of ABT-102, a potent and selective TRPV1 antagonist, enhances TRPV1-mediated analgesic activity in rodents, but attenuates antagonist-induced hyperthermia.
Honore P, Chandran P, Hernandez G, Gauvin DM, Mikusa JP, Zhong C, Joshi SK, Ghilardi JR, Sevcik MA, Fryer RM, Segreti JA, Banfor PN, Marsh K, Neelands T, Bayburt E, Daanen JF, Gomtsyan A, Lee CH, Kort ME, Reilly RM, Surowy CS, Kym PR, Mantyh PW, Sullivan JP, Jarvis MF, Faltynek CR. Honore P, et al. Pain. 2009 Mar;142(1-2):27-35. doi: 10.1016/j.pain.2008.11.004. Epub 2009 Jan 9. Pain. 2009. PMID: 19135797 - Contribution of acidic extracellular microenvironment of cancer-colonized bone to bone pain.
Yoneda T, Hiasa M, Nagata Y, Okui T, White F. Yoneda T, et al. Biochim Biophys Acta. 2015 Oct;1848(10 Pt B):2677-84. doi: 10.1016/j.bbamem.2015.02.004. Epub 2015 Feb 14. Biochim Biophys Acta. 2015. PMID: 25687976 Free PMC article. Review. - [Capsaicin receptor TRPV1].
Tominaga M. Tominaga M. Brain Nerve. 2008 May;60(5):493-501. Brain Nerve. 2008. PMID: 18516971 Review. Japanese.
Cited by
- Tumour-Derived Glutamate: Linking Aberrant Cancer Cell Metabolism to Peripheral Sensory Pain Pathways.
Fazzari J, Linher-Melville K, Singh G. Fazzari J, et al. Curr Neuropharmacol. 2017;15(4):620-636. doi: 10.2174/1570159X14666160509123042. Curr Neuropharmacol. 2017. PMID: 27157265 Free PMC article. - pH-Channeling in Cancer: How pH-Dependence of Cation Channels Shapes Cancer Pathophysiology.
Pethő Z, Najder K, Carvalho T, McMorrow R, Todesca LM, Rugi M, Bulk E, Chan A, Löwik CWGM, Reshkin SJ, Schwab A. Pethő Z, et al. Cancers (Basel). 2020 Sep 2;12(9):2484. doi: 10.3390/cancers12092484. Cancers (Basel). 2020. PMID: 32887220 Free PMC article. Review. - Cancer-nerve interplay in cancer progression and cancer-induced bone pain.
Yoneda T, Hiasa M, Okui T, Hata K. Yoneda T, et al. J Bone Miner Metab. 2023 May;41(3):415-427. doi: 10.1007/s00774-023-01401-6. Epub 2023 Jan 30. J Bone Miner Metab. 2023. PMID: 36715764 Review. - Capsaicin: A Two-Decade Systematic Review of Global Research Output and Recent Advances Against Human Cancer.
Adetunji TL, Olawale F, Olisah C, Adetunji AE, Aremu AO. Adetunji TL, et al. Front Oncol. 2022 Jul 13;12:908487. doi: 10.3389/fonc.2022.908487. eCollection 2022. Front Oncol. 2022. PMID: 35912207 Free PMC article. - Inhibition of p38-MAPK signaling pathway attenuates breast cancer induced bone pain and disease progression in a murine model of cancer-induced bone pain.
Sukhtankar D, Okun A, Chandramouli A, Nelson MA, Vanderah TW, Cress AE, Porreca F, King T. Sukhtankar D, et al. Mol Pain. 2011 Oct 20;7:81. doi: 10.1186/1744-8069-7-81. Mol Pain. 2011. PMID: 22014040 Free PMC article.
References
- Adami S (1997) Bisphosphonates in prostate carcinoma. Cancer 80: 1674-1679. - PubMed
- Berenson JR, Rosen LS, Howell A, Porter L, Coleman RE, Morley W, Dreicer R, Kuross SA, Lipton A, Seaman JJ (2001) Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer 91: 1191-1200. - PubMed
- Bevan S, Geppetti P (1994) Protons: small stimulants of capsaicin-sensitive sensory nerves. Trends Neurosci 17: 509-512. - PubMed
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816-824. - PubMed
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288: 306-313. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical