Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program - PubMed (original) (raw)
Clinical Trial
Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program
William C Knowler et al. Diabetes. 2005 Apr.
Abstract
The Diabetes Prevention Program (DPP) was a randomized clinical trial of prevention of type 2 diabetes in high-risk people. Troglitazone, an insulin-sensitizing agent, was used initially but was discontinued during the trial. Troglitazone therapy was compared with other DPP interventions, considering both the short-term "in-trial" results and the longer-term results after troglitazone were discontinued. From 1996 to 1998, participants were randomly assigned to treatment with metformin (n = 587), troglitazone (n = 585), double placebo (n = 582), or intensive lifestyle intervention (ILS) (n = 589). Because of concern regarding its liver toxicity, the troglitazone arm was discontinued in June 1998, after which follow-up of all participants continued. During the mean 0.9 year (range 0.5-1.5 years) of troglitazone treatment, the diabetes incidence rate was 3.0 cases/100 person-years, compared with 12.0, 6.7, and 5.1 cases/100 person-years in the placebo, metformin, and ILS participants (P < 0.001, troglitazone vs. placebo; P = 0.02, troglitazone vs. metformin; P = 0.18, troglitazone vs. ILS). This effect of troglitazone was in part due to improved insulin sensitivity with maintenance of insulin secretion. During the 3 years after troglitazone withdrawal, the diabetes incidence rate was almost identical to that of the placebo group. Troglitazone, therefore, markedly reduced the incidence of diabetes during its limited period of use, but this action did not persist. Whether other thiazolidinedione drugs used for longer periods can safely prevent diabetes remains to be determined.
Figures
FIG. 1
Mean values of body weight, waist circumference, and FPG and 2-h postload glucose concentrations in response to treatment. Sample sizes are shown in Table 2.
FIG. 2
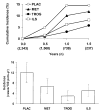
A: Cumulative incidence of diabetes during the time when troglitazone was used, according to treatment assignment. B: Diabetes incidence rates (cases/100 person-years) with 95% CIs during the time when troglitazone was used, according to treatment assignment.
FIG. 3
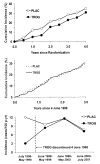
A: Cumulative incidence of diabetes (%) from date of randomization in participants assigned to placebo or troglitazone. B: Cumulative incidence of diabetes (%) from date of discontinuation of troglitazone (4 June 1998) in participants assigned to placebo or troglitazone. C: Diabetes incidence rates (cases/100 person-years) from date of randomization, showing the date of discontinuation of troglitazone (4 June 1998), in participants assigned to placebo or troglitazone.
Similar articles
- [Progress in the prevention of type 2 diabetes].
Schernthaner G. Schernthaner G. Wien Klin Wochenschr. 2003 Nov 28;115(21-22):745-57. doi: 10.1007/BF03040499. Wien Klin Wochenschr. 2003. PMID: 14743578 Review. German. - The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes.
The Diabetes Prevention Program Research Group. The Diabetes Prevention Program Research Group. Diabetes Care. 1999 Apr;22(4):623-34. doi: 10.2337/diacare.22.4.623. Diabetes Care. 1999. PMID: 10189543 Free PMC article. Clinical Trial. - An update on the Diabetes Prevention Program.
Ratner RE; Diabetes Prevention Program Research. Ratner RE, et al. Endocr Pract. 2006 Jan-Feb;12 Suppl 1(Suppl 1):20-4. doi: 10.4158/EP.12.S1.20. Endocr Pract. 2006. PMID: 16627375 Free PMC article. Review. - Troglitazone, an insulin action enhancer, improves glycaemic control and insulin sensitivity in elderly type 2 diabetic patients.
Kumar S, Prange A, Schulze J, Lettis S, Barnett AH. Kumar S, et al. Diabet Med. 1998 Sep;15(9):772-9. doi: 10.1002/(SICI)1096-9136(199809)15:9<772::AID-DIA677>3.0.CO;2-X. Diabet Med. 1998. PMID: 9737807 Clinical Trial. - The Pro12Ala variant at the peroxisome proliferator-activated receptor gamma gene and change in obesity-related traits in the Diabetes Prevention Program.
Franks PW, Jablonski KA, Delahanty L, Hanson RL, Kahn SE, Altshuler D, Knowler WC, Florez JC; Diabetes Prevention Program Research Group. Franks PW, et al. Diabetologia. 2007 Dec;50(12):2451-60. doi: 10.1007/s00125-007-0826-6. Epub 2007 Sep 27. Diabetologia. 2007. PMID: 17898990 Free PMC article. Clinical Trial.
Cited by
- Semaglutide and Cardiovascular Outcomes by Baseline HbA1c and Change in HbA1c in People With Overweight or Obesity but Without Diabetes in SELECT.
Lingvay I, Deanfield J, Kahn SE, Weeke PE, Toplak H, Scirica BM, Rydén L, Rathor N, Plutzky J, Morales C, Lincoff AM, Lehrke M, Jeppesen OK, Gajos G, Colhoun HM, Cariou B, Ryan D; SELECT Trial Investigators. Lingvay I, et al. Diabetes Care. 2024 Aug 1;47(8):1360-1369. doi: 10.2337/dc24-0764. Diabetes Care. 2024. PMID: 38907684 Free PMC article. Clinical Trial. - Effect of Semaglutide on Regression and Progression of Glycemia in People With Overweight or Obesity but Without Diabetes in the SELECT Trial.
Kahn SE, Deanfield JE, Jeppesen OK, Emerson SS, Boesgaard TW, Colhoun HM, Kushner RF, Lingvay I, Burguera B, Gajos G, Horn DB, Hramiak IM, Jastreboff AM, Kokkinos A, Maeng M, Matos ALSA, Tinahones FJ, Lincoff AM, Ryan DH; SELECT Trial Investigators. Kahn SE, et al. Diabetes Care. 2024 Aug 1;47(8):1350-1359. doi: 10.2337/dc24-0491. Diabetes Care. 2024. PMID: 38907683 Free PMC article. Clinical Trial. - Should Prediabetes be Treated Pharmacologically?
Davidson MB. Davidson MB. Diabetes Ther. 2023 Oct;14(10):1585-1593. doi: 10.1007/s13300-023-01449-7. Epub 2023 Jul 25. Diabetes Ther. 2023. PMID: 37490238 Free PMC article. - A Systematic Review and Meta-Analysis of Randomized Controlled Trials Comparing the Effects of Biguanides (Metformin) and Thiazolidinediones on Glucose Tolerance and Insulin Sensitivity in Patients With Type II Diabetes Mellitus.
Zaki HA, Iftikhar H, Shallik NA, Shaban E, Al-Marri NDR, Bashir I, Elhadad A, Zoghlami F, Abdalrubb A. Zaki HA, et al. Cureus. 2023 May 24;15(5):e39445. doi: 10.7759/cureus.39445. eCollection 2023 May. Cureus. 2023. PMID: 37362539 Free PMC article. Review. - The progression of secondary diabetes: A review of modeling studies.
Yang B, Li J, Haller MJ, Schatz DA, Rong L. Yang B, et al. Front Endocrinol (Lausanne). 2022 Dec 21;13:1070979. doi: 10.3389/fendo.2022.1070979. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36619543 Free PMC article. Review.
References
- Nolan JJ, Ludvik B, Beerdsen P, Joyce M, Olefsky J. Improvement in glucose tolerance and insulin resistance in obese subjects treated with troglitazone. N Engl J Med. 1994;331:1188–1193. - PubMed
- The Diabetes Prevention Program Research Group. The Diabetes Prevention Program: recruitment methods and results. Control Clin Trials. 2002;23:157–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical