A systems analysis of importin-{alpha}-{beta} mediated nuclear protein import - PubMed (original) (raw)
A systems analysis of importin-{alpha}-{beta} mediated nuclear protein import
Gregory Riddick et al. J Cell Biol. 2005.
Abstract
Importin-beta (Impbeta) is a major transport receptor for Ran-dependent import of nuclear cargo. Impbeta can bind cargo directly or through an adaptor such as Importin-alpha (Impalpha). Factors involved in nuclear transport have been well studied, but systems analysis can offer further insight into regulatory mechanisms. We used computer simulation and real-time assays in intact cells to examine Impalpha-beta-mediated import. The model reflects experimentally determined rates for cargo import and correctly predicts that import is limited principally by Impalpha and Ran, but is also sensitive to NTF2. The model predicts that CAS is not limiting for the initial rate of cargo import and, surprisingly, that increased concentrations of Impbeta and the exchange factor, RCC1, actually inhibit rather than stimulate import. These unexpected predictions were all validated experimentally. The model revealed that inhibition by RCC1 is caused by sequestration of nuclear Ran. Inhibition by Impbeta results from depletion nuclear RanGTP, and, in support of this mechanism, expression of mRFP-Ran reversed the inhibition.
Figures
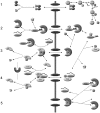
Figure 1.
Overview of the Impα–β nuclear protein import model (adapted in part from Catimel et al., 2001). (1) The NTF2 homodimer imports Ran into the nucleus. In the model, Ran can also translocate the NPC without NTF2, but with a much lower permeability constant. In the nucleus, RCC1 acts in a four-step reversible reaction to convert RanGDP to RanGTP. (2) Impα contains an auto-inhibitory region that prevents binding to an NLS. Upon binding to Impβ, the NH2-terminal auto-inhibitory domain folds into an α-helix, allowing NLS cargo to bind to Impα. Translocation of Impα–Impβ–cargo through the NPC is a reversible reaction, represented by a permeability constant. On the nuclear side of the NPC, RanGTP acts to dissociate Impβ from the complex. (3) The Impα–cargo heterodimer dissociates, assisted by high affinity binding of CAS–RanGTP to Impα. The RanGTP–Impβ complex translocates back to the cytoplasm where RanGAP (assisted by RanBP1 and Impα) catalyzes the hydrolysis of RanGTP to RanGDP, resulting in dissociation of Ran from the Impβ. (4) RanGTP–Cas–Impα complexes translocate back to the cytoplasm. RanGAP–RanBP1 hydrolyzes RanGTP to RanGDP. The complexes disassociate and transport receptors are recycled for another round of import. (5) Other association–dissociation reactions that occur in the nucleus. Note that passive diffusion by Ran, NLS-cargo, and Impα, and translocation of empty receptors through the NPC, are not depicted. Reversible reactions are represented by double-headed arrows. Essentially irreversible reactions are shown by a single-headed arrow. For simplicity, reactions involving the generic receptors and endogenous cargo, which are incorporated in the computer model, are not depicted here. The complete schematic is in Fig. S1. Concentrations and kinetic constants for the all components of the model are provided in Tables I–III.
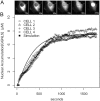
Figure 2.
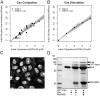
Nuclear import of GGNLS. (A) HeLA cells were cytoplasmically injected with 3 μM GGNLS. Cells were kept at 37°C in physiological saline for the duration of the experiment. Nuclear accumulation was quantified by time-lapse confocal microscopy. Images were taken every 20 s and pixel values within the nucleus were converted to concentration by the use of a standard series of known protein concentrations. (B) Results are overlaid with a simulation of identical conditions in the compartmental model.
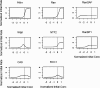
Figure 3.
Global sensitivity analysis of the computer model. Individual parameters in the model were varied over a five log scale, and initial rates of nuclear accumulation were calculated for each value. Graphs are normalized so that (0,1) indicates the original value used in the model.
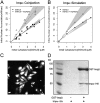
Figure 4.
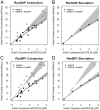
Effect of Impα-His6 on GGNLS nuclear import. (A) 20 μM GGNLS was microinjected with 5 μM Impα into the cytoplasm of HeLa cells maintained at 37°C and nuclear accumulation was recorded every 20 s. The initial concentration was calculated from the point at which nuclear and cytoplasmic GGNLS concentrations were equal, by comparison of mean pixel intensity in the cell with those of a series of protein standards. Initial rates were quantified as the slope of the import rate for the first 30–60 s of import. Data were plotted versus initial GGNLS concentration and fitted by linear regression analysis. Lines show the best fit to the data and gray areas indicate the 99% confidence limits of the slopes. Co-injection of Impα-His6 significantly increased the initial rate over baseline by two-way ANOVA (P = 0.00028). (B) The computer model was used to generate import data over the same range of GGNLS and Impα concentrations. Gray areas are taken from A. (C) Nuclear import of Impα. To verify the functionality of the His-tagged Impα the protein (1 mg/ml) was labeled with OGI and added to digitonin-permeabilized cells, which were then imaged by confocal microscopy. (D) Impα binds to Impβ. As a further test of functionality, 40 μg Impα-His6 was incubated with beads to which either 20 μg GST or 20 μg GST-Impβ had been attached. After extensive washing, bound proteins were analyzed by SDS-PAGE and stained with Coomassie blue.
Figure 5.
Effect of CAS on nuclear accumulation for GGNLS. (A) 20 μM GGNLS was microinjected with 5 μM recombinant CAS-His6 into the cytoplasm of HeLa cells and initial rates of nuclear import were measured and analyzed as in Fig. 4. (B) Initial rates were calculated from the computer model over the same range of GGNLS/CAS concentrations as in A. (C) To test the functionality of the recombinant CAS, the protein (1.3 mg/ml) was labeled as in Fig. 4 and incubated with digitonin-permeabilized cells. (D) Binding of CAS to Impα. 54 μg GST-Impα was attached to beads and incubated with 3 μg CAS ± 9 μg Ran(Q69)L-His6. After extensive washing the bound proteins were separated by SDS-PAGE and stained with Coomassie blue.
Figure 6.
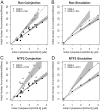
Effects of Ran and NTF2 on GGNLS import. Import assays were performed and analyzed as in Fig. 4, using recombinant Ran-His6 (A) or NTF2 (C). Co-injection of either Ran or NTF2 significantly increased the initial rate over baseline (P = 0.00016 for Ran; P = 0.000069 for NTF2). The functionality of these proteins has been described previously. Simulations were performed over the same ranges of concentration as in the experiments (B and D).
Figure 7.
Effects of RanGAP and RanBP1 on GGNLS import. Import assays were performed and analyzed as in Fig. 4, using recombinant GST-RanGAP (A) or GST-RanBP1 (C). The functionality of these proteins has been described previously. Simulations were performed over the same ranges of concentration as in the experiments (B and D). Co-injection of RanBP1 significantly increased the initial rate over baseline (P = 0.0022) but the effect of RanGAP fell under the threshold of significance (P = 0.067).
Figure 8.
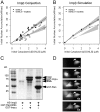
Effects of RCC1 on GGNLS import. HeLa cells were transiently transfected with pK-mRFP-RCC1 (A) or as a control with pK-RFP (B), and after 18 h were injected with GGNLS. Cells that visibly expressed the RCC1 and those that did not were both injected. Images show nuclear accumulation of GGNLS over time. (C) Cells expressing RFP-RCC1 accumulate more Ran in the nucleus. Cells were transfected as in A, then fixed and stained for endogenous Ran. RFP-RCC1 concentration was estimated to be 10–20 μM, based on standards using recombinant RFP protein (not depicted). Arrow indicates cell expressing RFP-RCC1
Figure 9.
Effects of Impβ on GGNLS import. (A) Import was measured and analyzed as in Fig. 4, using recombinant His6-Impβ. Co-injection of His6-Impβ significantly decreased the initial rate compared with baseline (P = 0.046). (B) Simulations were calculated as in Fig. 4. (C) Binding of His6-Impβ to GST-Impα and GST-Ran(Q69L). 54 μg GST-Impα and 21 μg GST-Ran(Q69L) were attached to beads and incubated with 60 μg His6-Impβ. After washing, the bound proteins were separated by SDS-PAGE and stained with Coomassie blue. (D) To test the functionality of the Impβ, the protein (15 mg/ml) was labeled as in Fig. 4, then injected into HeLa cells. Nuclear accumulation of the protein was monitored over time. Arrows indicate cell injected at time 0.
Figure 10.
The inhibition of GGNLS import by elevated Imp is caused by depletion of nuclear RanGTP. (Left) Simulation results show that excess Impβ deletes free RanGTP in the nucleus. Addition of Ran increases the total pool of free RanGTP in the nucleus and offsets the addition of Impβ. (Right) HeLA cells were transfected with pK-mRFP-Ran. After 12 h, cells were cytoplasmically microinjected with 20 μM GGNLS + 5 μM Impβ. Initial rates of GGNLS import were then quantified for both transfected cells that showed nuclear accumulation of RFP-Ran and nontransfected cells in the same population. Both experimental groups were measured over a range of initial GGNLS concentrations between 2 and 4 μM, and show a significant difference in mean initial rates (P < 0.002). Simulation results using 1 μM Impβ, 5 μM Ran, and 4 μM GGNLS are displayed next to experimental results.
Similar articles
- Importin-beta is a GDP-to-GTP exchange factor of Ran: implications for the mechanism of nuclear import.
Lonhienne TG, Forwood JK, Marfori M, Robin G, Kobe B, Carroll BJ. Lonhienne TG, et al. J Biol Chem. 2009 Aug 21;284(34):22549-58. doi: 10.1074/jbc.M109.019935. Epub 2009 Jun 23. J Biol Chem. 2009. PMID: 19549784 Free PMC article. - Intracellular calcium levels can regulate Importin-dependent nuclear import.
Kaur G, Ly-Huynh JD, Jans DA. Kaur G, et al. Biochem Biophys Res Commun. 2014 Jul 18;450(1):812-7. doi: 10.1016/j.bbrc.2014.06.047. Epub 2014 Jun 19. Biochem Biophys Res Commun. 2014. PMID: 24953690 - Plant importin alpha binds nuclear localization sequences with high affinity and can mediate nuclear import independent of importin beta.
Hübner S, Smith HM, Hu W, Chan CK, Rihs HP, Paschal BM, Raikhel NV, Jans DA. Hübner S, et al. J Biol Chem. 1999 Aug 6;274(32):22610-7. doi: 10.1074/jbc.274.32.22610. J Biol Chem. 1999. PMID: 10428841 - [Nucleocytoplasmic transport mediated by importin-β family members].
Kimura M. Kimura M. Seikagaku. 2015 Feb;87(1):7-15. Seikagaku. 2015. PMID: 26571549 Review. Japanese. No abstract available. - Nucleocytoplasmic transport: Ran, beta and beyond.
Kuersten S, Ohno M, Mattaj IW. Kuersten S, et al. Trends Cell Biol. 2001 Dec;11(12):497-503. doi: 10.1016/s0962-8924(01)02144-4. Trends Cell Biol. 2001. PMID: 11719056 Review.
Cited by
- Karyopherin Alpha 1 Regulates Satellite Cell Proliferation and Survival by Modulating Nuclear Import.
Choo HJ, Cutler A, Rother F, Bader M, Pavlath GK. Choo HJ, et al. Stem Cells. 2016 Nov;34(11):2784-2797. doi: 10.1002/stem.2467. Epub 2016 Aug 1. Stem Cells. 2016. PMID: 27434733 Free PMC article. - Is the Cell Nucleus a Necessary Component in Precise Temporal Patterning?
Albert J, Rooman M. Albert J, et al. PLoS One. 2015 Jul 30;10(7):e0134239. doi: 10.1371/journal.pone.0134239. eCollection 2015. PLoS One. 2015. PMID: 26226505 Free PMC article. - Stimulated nuclear import by β-like importins.
Flores K, Seger R. Flores K, et al. F1000Prime Rep. 2013 Oct 1;5:41. doi: 10.12703/P5-41. F1000Prime Rep. 2013. PMID: 24167722 Free PMC article. Review. - Intermolecular masking of the HIV-1 Rev NLS by the cellular protein HIC: novel insights into the regulation of Rev nuclear import.
Gu L, Tsuji T, Jarboui MA, Yeo GP, Sheehy N, Hall WW, Gautier VW. Gu L, et al. Retrovirology. 2011 Mar 14;8:17. doi: 10.1186/1742-4690-8-17. Retrovirology. 2011. PMID: 21401918 Free PMC article. - Regulator of Chromosome Condensation 2 Modulates Cell Cycle Progression, Tumorigenesis, and Therapeutic Resistance.
Guo K, Zhao C, Lang B, Wang H, Zheng H, Zhang F. Guo K, et al. Front Mol Biosci. 2021 Jan 13;7:620973. doi: 10.3389/fmolb.2020.620973. eCollection 2020. Front Mol Biosci. 2021. PMID: 33521058 Free PMC article. Review.
References
- Bäck, T., and H.-P. Schwefel. 1993. An overview of evolutionary algorithms for parameter optimization. Evol. Comput. 1:1–23. - PubMed
- Baker, R.P., M.T. Harreman, J.F. Eccleston, A.H. Corbett, and M. Stewart. 2001. Interaction between Ran and Mog1 is required for efficient nuclear protein import. J. Biol. Chem. 276:41255–41262. - PubMed
- Bayliss, R., T. Littlewood, and M. Stewart. 2000. Structural basis for the interaction between FxFG nucleoporin repeats and importin-beta in nuclear trafficking. Cell. 102:99–108. - PubMed
- Bischoff, F.R., and D. Görlich. 1997. RanBP1 is crucial for the release of RanGTP from importin β-related nuclear transport factors. FEBS Lett. 419:249–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous