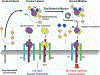Facilitated transport of a Dpp/Scw heterodimer by Sog/Tsg leads to robust patterning of the Drosophila blastoderm embryo - PubMed (original) (raw)
Facilitated transport of a Dpp/Scw heterodimer by Sog/Tsg leads to robust patterning of the Drosophila blastoderm embryo
Osamu Shimmi et al. Cell. 2005.
Erratum in
- Cell. 2005 May 6;121(3):493
Abstract
Patterning the dorsal surface of the Drosophila blastoderm embryo requires Decapentaplegic (Dpp) and Screw (Scw), two BMP family members. Signaling by these ligands is regulated at the extracellular level by the BMP binding proteins Sog and Tsg. We demonstrate that Tsg and Sog play essential roles in transporting Dpp to the dorsal-most cells. Furthermore, we provide biochemical and genetic evidence that a heterodimer of Dpp and Scw, but not the Dpp homodimer, is the primary transported ligand and that the heterodimer signals synergistically through the two type I BMP receptors Tkv and Sax. We propose that the use of broadly distributed Dpp homodimers and spatially restricted Dpp/Scw heterodimers produces the biphasic signal that is responsible for specifying the two dorsal tissue types. Finally, we demonstrate mathematically that heterodimer levels can be less sensitive to changes in gene dosage than homodimers, thereby providing further selective advantage for using heterodimers as morphogens.
Figures
Figure 1.
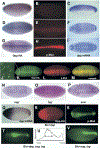
Dpp-HA Protein Forms a Dorsal Stripe during Cellularization of the Blastoderm Embryo (A-I) Dpp-HA staining in a Dpp-HA transgenic embryo (A, D, and G), p-Mad staining (B, E, and H), and in situ hybridization of dpp mRNA (C, F, and I) in a wt embryo. Early (A-C), middle (D-F), and late (G-I) blastoderm stages, lateral view (A-C) and dorsal view (D-I). Note that Dpp protein is localized in the dorsal half of embryo at the early blastoderm stage, as is dpp mRNA. As cellularization proceeds, Dpp protein is concentrated at the dorsal midline. Also note that Dpp protein accumulates dorsally with the same kinetics that p-Mad staining does. (J-M) Early gastrulation embryos double stained for Dpp HA (J and K, green) and pMad (L, red). Dpp-HA is seen in numerous punctate structures ([J], high-magnification view of boxed area in [K]). (N-P) Dorsal view of Dpp-HA staining in sog (N), tsg (O), and scw (P) mutant embryos. No localization of dpp-HA into a dorsal stripe is observed. Instead, staining remains broad throughout the dorsal region. (Q) Lateral view of dpp mRNA in Gal4-Bic>UAS-_dpp_-HA embryo. Strong expression of dpp was induced in the anterior end and endogenous dpp mRNA can be seen in the posterior part of embryo. (R) Dorsal view of HA staining in Gal4-Bic>UAS-_dpp_-HA embryo. Dpp-HA forms a dorsal stripe even though _dpp_-HA is expressed only in the anterior end. (S) Dorsal view of p-Mad staining in the same embryo of (R). (T) Dorsal view of Gal4-Bic > UAS-dpp, sog, tsg. (U) The p-Mad image of dorsal-most five cells in (D) was analyzed using ImageJ. (V) Dorsal view of Gal4-Bic>UAS-sog, tsg. The anterior half of p-Mad staining is lost.
Figure 2.
Dpp and Scw Form Heterodimers (A) HA-tagged Dpp and Flag-tagged Scw were coexpressed in S2 cells, and the conditioned medium was purified through anti-Flag M2 column. Conditioned medium (Sup), flowthrough fraction (FT), and eluted fraction by Flag peptide (Elution) were blotted and probed with anti-HA and anti-Flag antibodies. Dpp-HA was eluted from the Flag affinity column together with Scw-Flag. (B) A mixture of Dpp-HA and Scw-Flag homodimers were loaded and eluted from the Flag affinity column. All the Dpp-HA was in the flowthrough. (C) Purified Dpp-HA/Scw-Flag complex was analyzed by nonreduced (lanes 1 and 3) and reduced conditions (lanes 2 and 4). These gels were blotted and probed with anti-HA (lanes 1 and 2) and anti-Flag antibodies (lanes 3 and 4). The monomers of Dpp-HA and Scw-Flag show the distinct molecular weights of 25 kDa and 22 kDa, respectively (lanes 2 and 4); however, Dpp-HA/Scw-Flag shows exactly the same molecular weight of 39 kDa under nonreducing conditions (lanes 1 and 3), indicating that Dpp-HA and Scw-Flag form heterodimers. (D) Coimmunoprecipitation of Dpp-HA and Scw in the embryo. One to three hour embryos of control (yw flies) or Dpp-HA transgenic flies were collected, and the protein extracts were precipitated with anti-HA antibody-coupled beads, then analyzed by Western blotting using anti-HA and anti-Scw antibodies. Scw protein came down together with Dpp-HA, indicating that Dpp and Scw form heterodimers in the embryo. The double Dpp band is caused by cleavage of the pro-protein at either one of two different maturation sites (O.S. and M.B.O., unpublished data).
Figure 3.
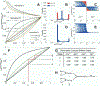
Dpp/Scw Heterodimer Shows Enhanced Signaling Compared to Homodimers and Enhanced Interactions with Sog and Tsg (A) Comparisons of BMP activities of each ligand in a cell-based signaling assay. BMP signalings of equivalent amounts of Dpp homodimer, Scw homodimer, Dpp/Scw heterodimer, or a mixture of Dpp and Scw homodimers were measured. Dpp/Scw heterodimer has approximately 10-fold higher activity than Dpp homodimers. Scw homodimers produce only low-level signals, and a mixture of Dpp and Scw homodimers showed an activity similar to Dpp homodimers alone. (B) Sog and Tsg synergistically inhibit Dpp/Scw heterodimer signaling. Dpp/Scw were preincubated with Sog and/or Tsg, and their BMP activities were measured. (C) Determination of receptor requirements for homo- and heterodimer signaling by RNAi analysis. Dpp homodimer signals primarily through Tkv as a type I receptor; in contrast, synergistic signaling by Dpp/Scw heterodimer requires both Tkv and Sax as type I receptors. (D) Coimmunoprecipitation of Tsg and/or Sog with ligands. Dpp/Scw heterodimer binds with higher affinity to Sog and Tsg than does either homodimer. (E) Comparisons of each ligand as a catalyst for Sog cleavage by Tld.
Figure 4.
Analysis of Target Gene Activation in dpp and scw Mutant Backgrounds (A-C) A dorsal view of pnr mRNA at blastoderm stage. Wild-type (A), scw mutant (B), and dpp mutant embryos (C). pnr is expressed in scw but not dpp mutant embryos. (D-F) Dorsal view of race mRNA in wild-type embryo (D), scw mutant (E), and dpp mutant embryos (F). race is not expressed in either scw or dpp mutant embryo.
Figure 5.
Analysis of Dimerization Reactions
Figure 6.
Heterodimer Formation Compensates for Reductions of Input (A) Ratio of dimer output for reductions of a monomer input. Heterodimer, homodimer X, and homodimer Y are shown for Ω = 1/2, and β = 0.2 (black), 1 (green), or 5 (orange). An example calculation is shown for a 50% (λ = 1/2) reduction of Scw and for Dpp. (B and C) Histogram and β dependence of computational results of 10,000 simulations with randomly chosen parameter values for Ω ≠ 1/2. Red data correspond to solutions in which heterodimer formation is dominant (Ω > 1/2), and blue data are for homodimer-dominant processes (Ω < 1/2). A green line for Ω = 1/2 is shown in (C) for reference. (D and E) Same as (B-(C) except for Sog/Tsg case with linear degradation and no homodimer formation. (F-H) Binding cascade enhances robustness to reductions of input monomer. (F) Heterodimer ratios for Dpp/Scw (black) and Sog/Tsg complex (green) are shown for Ω = 1/2, β = 0.2 (solid), and β = 5 (dotted). An example dimerization and binding cascade (solid red line) leading to robust BMP signaling is shown for the case of tsg+/− embryos. This demonstrates graphically the enhanced buffering obtained by the sequence of binding reactions shown in (H). The perturbation λ and output from each stage are shown on the right of (F). Example output results for each stage are shown in (G) for scw+/−, dpp+/−, sog+/−, and tsg+/− mutants for given values of Ω = 1/2, β = 0.2, 5.
Figure 7.
Schematic Model for Patterning Dorsal Tissues in the Drosophila Embryo Homodimers and heterodimers of Dpp and Scw are produced throughout the dorsal domain. Sog is expressed and secreted in the ventral lateral region and diffuses toward the dorsal side. Dpp and Scw homodimers do not bind to Sog and Tsg with high affinity. As a result, they are free to bind with receptors, but they produce only low-level signals that can activate targets such as pnr. In contrast, Dpp/Scw heterodimers bind with high affinity to Sog and Tsg. Net flux of the complex toward the dorsal midline leads to an increase in the heterodimer concentration at the midline. Tld processes Sog at the midline to release the ligand, which then binds to a receptor complex containing both Sax and Tkv. This complex produces a synergistic high signal that activates high-level response genes such as race and leads to specification of the amnioserosa.
Similar articles
- Interpretation of a BMP activity gradient in Drosophila embryos depends on synergistic signaling by two type I receptors, SAX and TKV.
Nguyen M, Park S, Marqués G, Arora K. Nguyen M, et al. Cell. 1998 Nov 13;95(4):495-506. doi: 10.1016/s0092-8674(00)81617-7. Cell. 1998. PMID: 9827802 - Spatially restricted activation of the SAX receptor by SCW modulates DPP/TKV signaling in Drosophila dorsal-ventral patterning.
Neul JL, Ferguson EL. Neul JL, et al. Cell. 1998 Nov 13;95(4):483-94. doi: 10.1016/s0092-8674(00)81616-5. Cell. 1998. PMID: 9827801 - Biphasic activation of the BMP pathway patterns the Drosophila embryonic dorsal region.
Dorfman R, Shilo BZ. Dorfman R, et al. Development. 2001 Mar;128(6):965-72. doi: 10.1242/dev.128.6.965. Development. 2001. PMID: 11222150 - BMP signalling: synergy and feedback create a step gradient.
Ashe HL. Ashe HL. Curr Biol. 2005 May 24;15(10):R375-7. doi: 10.1016/j.cub.2005.05.003. Curr Biol. 2005. PMID: 15916937 Review. - Differing strategies for forming the arthropod body plan: lessons from Dpp, Sog and Delta in the fly Drosophila and spider Achaearanea.
Oda H, Akiyama-Oda Y. Oda H, et al. Dev Growth Differ. 2008 May;50(4):203-14. doi: 10.1111/j.1440-169X.2008.00998.x. Dev Growth Differ. 2008. PMID: 18366383 Review.
Cited by
- Fine-tuned shuttles for bone morphogenetic proteins.
Wharton KA, Serpe M. Wharton KA, et al. Curr Opin Genet Dev. 2013 Aug;23(4):374-84. doi: 10.1016/j.gde.2013.04.012. Epub 2013 Jun 2. Curr Opin Genet Dev. 2013. PMID: 23735641 Free PMC article. Review. - Morphogen transport.
Müller P, Rogers KW, Yu SR, Brand M, Schier AF. Müller P, et al. Development. 2013 Apr;140(8):1621-38. doi: 10.1242/dev.083519. Development. 2013. PMID: 23533171 Free PMC article. - Alterations in the Smad pathway in human cancers.
Samanta D, Datta PK. Samanta D, et al. Front Biosci (Landmark Ed). 2012 Jan 1;17(4):1281-93. doi: 10.2741/3986. Front Biosci (Landmark Ed). 2012. PMID: 22201803 Free PMC article. Review. - BMP7 functions predominantly as a heterodimer with BMP2 or BMP4 during mammalian embryogenesis.
Kim HS, Neugebauer J, McKnite A, Tilak A, Christian JL. Kim HS, et al. Elife. 2019 Sep 30;8:e48872. doi: 10.7554/eLife.48872. Elife. 2019. PMID: 31566563 Free PMC article. - N-linked glycosylation restricts the function of Short gastrulation to bind and shuttle BMPs.
Negreiros E, Herszterg S, Kang KH, Câmara A, Dias WB, Carneiro K, Bier E, Todeschini AR, Araujo H. Negreiros E, et al. Development. 2018 Nov 19;145(22):dev167338. doi: 10.1242/dev.167338. Development. 2018. PMID: 30355725 Free PMC article.
References
- Arora K, Levine MS, and O’Connor MB (1994). The screw gene encodes a ubiquitously expressed member of the TGF-beta family required for specification of dorsal cell fates in the Drosophila embryo. Genes Dev. 8, 2588–2601. - PubMed
- Ashe HL, Mannervik M, and Levine M (2000). Dpp signaling thresholds in the dorsal ectoderm of the Drosophila embryo. Development 127, 3305–3312. - PubMed
- Biehs B, Francois V, and Bier E (1996). The Drosophila short gastrulation gene prevents Dpp from autoactivating and suppressing neurogenesis in the neuroectoderm. Genes Dev. 10, 2922–2934. - PubMed
- Decotto E, and Ferguson EL (2001). A positive role for Short gastrulation in modulating BMP signaling during dorsoventral patterning in the Drosophila embryo. Development 128, 3831–3841. - PubMed
- Dorfman R, and Shilo BZ (2001). Biphasic activation of the BMP pathway patterns the Drosophila embryonic dorsal region. Development 128, 965–972. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous