Phosphorylation of p21 in G2/M promotes cyclin B-Cdc2 kinase activity - PubMed (original) (raw)
Phosphorylation of p21 in G2/M promotes cyclin B-Cdc2 kinase activity
Bipin C Dash et al. Mol Cell Biol. 2005 Apr.
Abstract
Little is known about the posttranslational control of the cyclin-dependent protein kinase (CDK) inhibitor p21. We describe here a transient phosphorylation of p21 in the G2/M phase. G2/M-phosphorylated p21 is short-lived relative to hypophosphorylated p21. p21 becomes nuclear during S phase, prior to its phosphorylation by CDK2. S126-phosphorylated cyclin B1 binds to T57-phosphorylated p21. Cdc2 kinase activation is delayed in p21-deficient cells due to delayed association between Cdc2 and cyclin B1. Cyclin B1-Cdc2 kinase activity and G2/M progression in p21-/- cells are restored after reexpression of wild-type but not T57A mutant p21. The cyclin B1 S126A mutant exhibits reduced Cdc2 binding and has low kinase activity. Phosphorylated p21 binds to cyclin B1 when Cdc2 is phosphorylated on Y15 and associates poorly with the complex. Dephosphorylation on Y15 and phosphorylation on T161 promotes Cdc2 binding to the p21-cyclin B1 complex, which becomes activated as a kinase. Thus, hyperphosphorylated p21 activates the Cdc2 kinase in the G2/M transition.
Figures
FIG. 1.
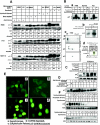
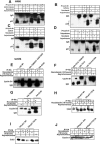
p21 is phosphorylated at G2/M phase. (A) Expression of the p21 protein in asynchronous and synchronized cells at G1, S, or G2/M phase. Western blot using H460, PA1, or HCT116 cells with p21 monoclonal antibody showed a high-molecular-mass band of ∼23-kDa p21 protein at G2/M (nocodazole for 18 h; see Materials and Methods) but a reduced phosphorylated protein band postexposure to UV or MMC for anadditional 4 h. (B) (a) Phosphatase treatment of G2/M-enriched cells abolished the high-molecular-weight p21 band. (b) 32P-Orthophosphate-labeled asynchronous or nocodazole-arrested H460 cells showed phosphorylated p21 protein after immunoprecipitation with p21 antibody. (c) Nocodazole-treated plus 32P-orthophosphate-labeled cells showed hyper- and hypophosphorylated bands after 2D gel electrophoresis. Similarly, Western blotting also showed two bands separated according to their isoelectric points. Asynchronous cells showed a single p21 band after 2D-gel electrophoresis. (d) Asynchronous or nocodazole-treated DLD1 p21−/− cell extracts were incubated in vitro with purified His-tagged p21 in the presence of [γ-32P]ATP. High phosphorylation of His-p21 was observed after immunoprecipitation with nickel-nitrilotriacetic acid, using cell extract from nocodazole-treated DLD1 p21−/− cells. (C) Treatment of H460 cells with other spindle poisoning agents, including taxol, vincristine, colchicine, or colcimid (like nocodazole) enriched for cells with hyperphosphorylated p21. (D) H460 cells released from aphidicolin block had a peak of S-phase cells at 4 h and showed phosphorylation of p21 at 8 h (corresponding to G2/M-enriched cells). Hyperphosphorylated p21 disappeared at subsequent times with progression out of the G2/M phase. (E) (a) In asynchronous cells, p21 localized in the cytoplasm in the majority of H460 cells with some cells showing nuclear localization (approximately 20 to 25%). (b) In FBS-depleted G1-arrested cells, p21 showed only a cytoplasmic localization. (c) In aphidicolin-released cells, the p21 protein appeared to translocate to the nucleus, and similarly, in nocodazole-arrested G2/M cells, the p21 antibodies stained nuclear p21 (d). (F) 18-h nocodazole-treated G2/M-enriched H460 cells were exposed to cycloheximide (10 μg/ml) for different time periods. The half-life of the p21 phosphorylated band was less than that of the unphosphorylated band (upper panels). Cycloheximide-treated asynchronous cells also showed a pattern of protein elimination for the unphosphorylated p21 band similar to that observed with the nocodazole-treated cells (lower panels).
FIG.2.
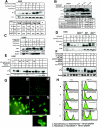
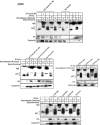
CDK2 is the principal kinase that phosphorylates p21 at G2/M phase. (A) Ten, 20, and 40 μM concentrations of butyrolactone dissolved in dimethyl sulfoxide were added, along with nocodazole (0.8 μg/ml), to H460 cells and incubated at 37°C for 18 h with 5% CO2. Western analysis shows complete dephosphorylation of p21 even at the lowest concentrations of butyrolactone used. (B) Transient transfection of wild-type CDK2 or DN-CDK2 in U2OS cells showed reduced phosphorylation of p21 at Nocodazole-arrested G2/M phase. Cell cycle profile of wild-type CDK2 or DN-CDK2-transfected arrested U20S cells in G2/M phase after treatment with nocodazole for 18 h. (C) Transient transfection of pSuper Retro CDK2 RNAi in U2OS cells showed reduced CDK2 expression in asynchronous or nocodazole-treated cells and also inhibited phosphorylation of p21 in G2/M-enriched cells. However, no change in CDK2 levels or p21 phosphorylation was observed in control vector-transfected cells. (D) Caffeine, an ATM/ATR kinase inhibitor, inhibits p21 phosphorylation when added at 2 or 4 mM concentrations to the exponentially growing H460 cells at 6 h before or at the same time as nocodazole but not if added after 6 h of nocodazole treatment. (E) Two different concentrations (50 or 100 μM) of a 24-amino-acid p21 peptide including the NLS/PCNA region (see Materials and Methods for sequence) dissolved in water were added to asynchronous H460 cells without FBS for 3 h, followed by 0.8-μg/ml nocodazole with complete medium and incubation for another 18 h. Cells were harvested and dissolved in whole-cell lysis buffer, electrophoresed on SDS-PAGE, and immunoblotted with p21 antibodies. There was almost complete inhibition of p21 phosphorylation with or without UV exposure in nocodazole-treated cells at the higher dose, while less inhibition was observed at the lower dose tested. (F) H460 cells were FBS depleted for 30 h, followed by aphidicolin treatment for another 24 h and release from aphidicolin block for 8 or 10 h. p21 peptide was added after 4 h of aphidicolin release without FBS for 1 h to adsorb the peptide, with the cells subjected to 10% FBS added and incubated at 37°C for another 4 or 6 h before cells were harvested. Western analysis showed dephosphorylation of p21 protein after 8 or 10 h of release from aphidicolin with the 100 μM peptide-treated cells. (G) Immunofluorescence staining for p21 protein expression in H460 cells treated with p21 peptide with or without nocodazole. Nuclear p21 protein translocation was blocked by the p21 peptide with evidence cytoplasmic localization in p21- plus nocodazole-treated H460 cells (d) but not in nocodazole-treated cells in the absence of peptide (c). The asynchronous H460 cells showed cytoplasmic or nuclear localization of the protein (a). p21 protein was detected throughout the cells in p21 peptide-treated cells not exposed to nocodazole (b). Inset shows an enlarged view of cells from panel G-d. (H) Treatment with p21 peptide along with nocodazole in H460 and HCT116 cells resulted in a significant population of annexin V-positive cells in both cell lines (panels H-d) compared to the controls (panels H-a, H-b, and H-c).
FIG. 3.
Phosphorylation of p21 precedes G2/M progression. (A) H460 cells were treated with nocodazole (0.8 μg/ml) for different time points (as indicated) and harvested. Samples were electrophoresed by using SDS-15% PAGE, and membranes were immunoblotted with different antibodies to detect p21, Cdc2, CDK2, cyclin B1, cyclin A, Cdc25C, PCNA, and actin (as indicated). Phosphorylation of p21 was observed after 6 h of nocodazole treatment, and this increased till 18 h, where equal intensities of hyperphosphorylated and hypophosphorylated bands were noted between 15 to 18 h. Gradually the hyperphosphorylated band intensity decreased at subsequent time points but persisted till 48 h. The Cdc2 and cyclin B1 band intensity became stronger after 12 h of nocodazole treatment and undetectable in the Western blots after 36 h of nocodazole treatment. The mitotic form of Cdc25C appeared after 9 h of nocodazole treatment and persisted till 36 h of nocodazole exposure. The level of CDK2 did not appear to change significantly with nocodazole treatment. PCNA expression also remained essentially unchanged throughout the prolonged period of nocodazole treatment. Actin was used as a loading control. Blots were probed with anti-Cdc2-Y15 antibodies in nocodazole-treated cells, and this revealed strong bands of inactive Y15-phosphorylated protein till 6 h after nocodazole exposure, and subsequently Cdc2 phosphorylated at Y15 diminished up to 36 h, with reappearance of a strong banding pattern by 48 h. Cell cycle distribution is depicted in the chart at the bottom of panel A. BrdU incorporation as an indicator of S phase, in nocodazole-treated H460 cells, showed complete loss of S phase with increase in G2/M-phase cells after 18 h. Similar results were observed in cells treated with nocodazole alone compared to those with nocodazole- plus BrdU-treated cells. (B) Delayed S-G2 and G2/M progression in p21−/− cells versus p21+/+ cells. Analysis of cell cycle profile after nocodazole treatment for HCT116 and DLD1 cells demonstrated that the change in profile from that of asynchronous cells took 3 h for p21+/+ cells, whereas for the p21−/− cells it took 6 h. FACS analysis also showed a higher number of cell deaths at longer time periods of nocodazole treatment for p21−/− cells than for p21+/+ cells. Cell cycle analysis is shown for the entire time course in the table below the histograms. AS refers to asynchronous cells. (C) Comparison of p21+/+ and p21−/− cells released from aphidicolin block at different time points showed appearance of p21 phosphorylation as early as 3 h and maximum phosphorylation at 6 to 8 h after release, coinciding well with an increased percentage of G2/M cells. Subsequently, the phosphorylated p21 disappeared as the cells progressed to the subsequent cell phase.
FIG. 3.
Phosphorylation of p21 precedes G2/M progression. (A) H460 cells were treated with nocodazole (0.8 μg/ml) for different time points (as indicated) and harvested. Samples were electrophoresed by using SDS-15% PAGE, and membranes were immunoblotted with different antibodies to detect p21, Cdc2, CDK2, cyclin B1, cyclin A, Cdc25C, PCNA, and actin (as indicated). Phosphorylation of p21 was observed after 6 h of nocodazole treatment, and this increased till 18 h, where equal intensities of hyperphosphorylated and hypophosphorylated bands were noted between 15 to 18 h. Gradually the hyperphosphorylated band intensity decreased at subsequent time points but persisted till 48 h. The Cdc2 and cyclin B1 band intensity became stronger after 12 h of nocodazole treatment and undetectable in the Western blots after 36 h of nocodazole treatment. The mitotic form of Cdc25C appeared after 9 h of nocodazole treatment and persisted till 36 h of nocodazole exposure. The level of CDK2 did not appear to change significantly with nocodazole treatment. PCNA expression also remained essentially unchanged throughout the prolonged period of nocodazole treatment. Actin was used as a loading control. Blots were probed with anti-Cdc2-Y15 antibodies in nocodazole-treated cells, and this revealed strong bands of inactive Y15-phosphorylated protein till 6 h after nocodazole exposure, and subsequently Cdc2 phosphorylated at Y15 diminished up to 36 h, with reappearance of a strong banding pattern by 48 h. Cell cycle distribution is depicted in the chart at the bottom of panel A. BrdU incorporation as an indicator of S phase, in nocodazole-treated H460 cells, showed complete loss of S phase with increase in G2/M-phase cells after 18 h. Similar results were observed in cells treated with nocodazole alone compared to those with nocodazole- plus BrdU-treated cells. (B) Delayed S-G2 and G2/M progression in p21−/− cells versus p21+/+ cells. Analysis of cell cycle profile after nocodazole treatment for HCT116 and DLD1 cells demonstrated that the change in profile from that of asynchronous cells took 3 h for p21+/+ cells, whereas for the p21−/− cells it took 6 h. FACS analysis also showed a higher number of cell deaths at longer time periods of nocodazole treatment for p21−/− cells than for p21+/+ cells. Cell cycle analysis is shown for the entire time course in the table below the histograms. AS refers to asynchronous cells. (C) Comparison of p21+/+ and p21−/− cells released from aphidicolin block at different time points showed appearance of p21 phosphorylation as early as 3 h and maximum phosphorylation at 6 to 8 h after release, coinciding well with an increased percentage of G2/M cells. Subsequently, the phosphorylated p21 disappeared as the cells progressed to the subsequent cell phase.
FIG. 4.
Absence of p21 in HCT116 and DLD1 cells delays cyclin B1-Cdc2 kinase activation. (A) Immnodepletion with cyclin B1 monoclonal antibodies in p21+/+ and p21−/− cell lysates collected after Nocodazole treatment at different time points was followed by an in vitro histone H1 kinase assay. The results demonstrate that the cyclin B1-associated kinase activity begins to rise at 6 h in HCT116 p21+/+ cells but is significantly delayed in p21−/− cells. In DLD1 p21+/+ cells, the H1 kinase activity also appears earlier than in p21−/− cells. Kinase activity was also observed to persist for 6 to 8 h longer following nocodazole exposure of HCT116 and DLD1 p21−/− cells than with the matched p21+/+ cells. Association between immunoprecipitated cyclin B1 and Cdc2 or T161-phosphorylated Cdc2 is shown. (B) Delayed histone H1 kinase activity was noted in aphidicolin-released cells immunodepleted with cyclin B1 antibody in p21−/− cells. Surprisingly, in DLD1 p21+/+ cells the kinase activity not only started at early time points but also progressed for longer time periods. However, the nocodazole exposure (A) or aphidicolin release (B) methods gave similar results with respect to the delay in cyclin B1-associated histone H1 kinase activation in the two different p21−/− human cell lines. Association between immunoprecipitated cyclin B1 and Cdc2- or T161-phosphorylated Cdc2 is shown. In the lower panels, association between p21- and T161-phosphorylated but not Y15-phosphorylated Cdc2 is shown. (C) Association of kinase activity with p21-containing immune complexes from cells enriched at G2/M. Nocodazole-treated G2/M-enriched H460 cells immunodepleted with p21 antibodies showed H1 histone phosphorylation. (D) CDK2-associated kinase activity is observed at all progressive time points following Nocodazole exposure with a peak at 15 to 18 h in p21+/+ cells (HCT116 and DLD1). After 18 h, the kinase activity returned to the basal levels in HCT116 and DLD1 p21−/− cells.
FIG. 4.
Absence of p21 in HCT116 and DLD1 cells delays cyclin B1-Cdc2 kinase activation. (A) Immnodepletion with cyclin B1 monoclonal antibodies in p21+/+ and p21−/− cell lysates collected after Nocodazole treatment at different time points was followed by an in vitro histone H1 kinase assay. The results demonstrate that the cyclin B1-associated kinase activity begins to rise at 6 h in HCT116 p21+/+ cells but is significantly delayed in p21−/− cells. In DLD1 p21+/+ cells, the H1 kinase activity also appears earlier than in p21−/− cells. Kinase activity was also observed to persist for 6 to 8 h longer following nocodazole exposure of HCT116 and DLD1 p21−/− cells than with the matched p21+/+ cells. Association between immunoprecipitated cyclin B1 and Cdc2 or T161-phosphorylated Cdc2 is shown. (B) Delayed histone H1 kinase activity was noted in aphidicolin-released cells immunodepleted with cyclin B1 antibody in p21−/− cells. Surprisingly, in DLD1 p21+/+ cells the kinase activity not only started at early time points but also progressed for longer time periods. However, the nocodazole exposure (A) or aphidicolin release (B) methods gave similar results with respect to the delay in cyclin B1-associated histone H1 kinase activation in the two different p21−/− human cell lines. Association between immunoprecipitated cyclin B1 and Cdc2- or T161-phosphorylated Cdc2 is shown. In the lower panels, association between p21- and T161-phosphorylated but not Y15-phosphorylated Cdc2 is shown. (C) Association of kinase activity with p21-containing immune complexes from cells enriched at G2/M. Nocodazole-treated G2/M-enriched H460 cells immunodepleted with p21 antibodies showed H1 histone phosphorylation. (D) CDK2-associated kinase activity is observed at all progressive time points following Nocodazole exposure with a peak at 15 to 18 h in p21+/+ cells (HCT116 and DLD1). After 18 h, the kinase activity returned to the basal levels in HCT116 and DLD1 p21−/− cells.
FIG. 4.
Absence of p21 in HCT116 and DLD1 cells delays cyclin B1-Cdc2 kinase activation. (A) Immnodepletion with cyclin B1 monoclonal antibodies in p21+/+ and p21−/− cell lysates collected after Nocodazole treatment at different time points was followed by an in vitro histone H1 kinase assay. The results demonstrate that the cyclin B1-associated kinase activity begins to rise at 6 h in HCT116 p21+/+ cells but is significantly delayed in p21−/− cells. In DLD1 p21+/+ cells, the H1 kinase activity also appears earlier than in p21−/− cells. Kinase activity was also observed to persist for 6 to 8 h longer following nocodazole exposure of HCT116 and DLD1 p21−/− cells than with the matched p21+/+ cells. Association between immunoprecipitated cyclin B1 and Cdc2 or T161-phosphorylated Cdc2 is shown. (B) Delayed histone H1 kinase activity was noted in aphidicolin-released cells immunodepleted with cyclin B1 antibody in p21−/− cells. Surprisingly, in DLD1 p21+/+ cells the kinase activity not only started at early time points but also progressed for longer time periods. However, the nocodazole exposure (A) or aphidicolin release (B) methods gave similar results with respect to the delay in cyclin B1-associated histone H1 kinase activation in the two different p21−/− human cell lines. Association between immunoprecipitated cyclin B1 and Cdc2- or T161-phosphorylated Cdc2 is shown. In the lower panels, association between p21- and T161-phosphorylated but not Y15-phosphorylated Cdc2 is shown. (C) Association of kinase activity with p21-containing immune complexes from cells enriched at G2/M. Nocodazole-treated G2/M-enriched H460 cells immunodepleted with p21 antibodies showed H1 histone phosphorylation. (D) CDK2-associated kinase activity is observed at all progressive time points following Nocodazole exposure with a peak at 15 to 18 h in p21+/+ cells (HCT116 and DLD1). After 18 h, the kinase activity returned to the basal levels in HCT116 and DLD1 p21−/− cells.
FIG.5.
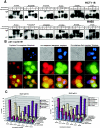
Cyclin B1 interacts exclusively with hyperphosphorylated p21, whereas Cdc2 interacts preferentially with hyperphosphorylated p21 compared to hypo-phosphorylated p21. (A) Immunodepletion of asynchronous or G2/M-enriched H460 or HCT116 cells with various monoclonal antibodies (as indicated) and immunoblotting for p21 protein. Total cell extracts from asynchronous and nocodazole-treated cells are shown in each case to mark the migration of the hyper- and hypophosphorylated p21. Immunoprecipitation using anti-cyclin A, CDK2, or PCNA antibodies revealed similar association of these proteins with hyper- and hypo-phosphorylated p21. The p21 monoclonal antibody used for immunoprecipitation (rightmost panels) bound and precipitated both the hyper- and hypophosphorylated p21 proteins. (B) p21 colocalizes with cyclin B1 in the nucleus at G2/M. Eighteen-hour nocodazole-treated H460 cells released from microtubule inhibition for 0.5 h were fixed and simultaneously stained with human monoclonal p21 (red) and human cyclin B1 polyclonal antibodies (green). Prophase, prometaphase, and metaphase cells show milkish/pink granular structures over the chromatin material with nuclear p21 and cyclin B1 localization after cells were treated for 18 h with nocodazole or released from nocodazole for half an hour. In late anaphase or early telophase stage, only p21 was localized in the nucleoplasm (red), but cyclin B1 degraded. (C) Phosphorylated p21 acts as a positive regulator of the G2-prophase stage. Comparison of percentage of mitotic phases in nocodazole-treated or nocodazole-released cells at different time points using DLD1 p21−/− and p21+/+ cells is shown.
FIG.6.
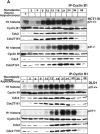
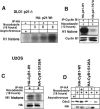
Cyclin B1 interacts specifically with phosphorylated Thr 57 of p21. (A) H460 cell lysates treated with nocodazole plus a GSK3 inhibitor (10 μM) and immunodepleted with anti-cyclin B1 antibody showed hyperphosphorylated p21 on the immunoblot. (B) Caffeine- plus nocodazole-treated cell lysates showed hyperphosphorylated p21 after immunodepletion with anti-cyclin B1 antibodies. Even though caffeine inhibits phosphorylation of p21 (Fig. 2D and F), residual hyperphosphorylated p21 immunoprecipitates with cyclin B1. (C) UCN-01, a PKC and Chk1 kinase inhibitor, did not prevent anti-cyclin B1 from immunoprecipitating the hyperphosphorylated p21 band in nocodazole-treated G2/M-arrested H460 cells. (D) Butyrolactone, the CDK inhibitor, blocked the interaction between cyclin B1 and p21 in G2/M-enriched nocodazole-treated cells. (E) Transient transfection of HA-tagged p21HA-Wt, p21HA-T98A, S99A mutant, or p21HA-S130A mutant in U20S cells for 24 h and treatment with nocodazole for 18 h did not prevent interaction between phosphorylated p21 and cyclin B1. (F) p21HA-T57A or p21HA-T57A,T98A, S99A mutant, or p21HAT57A,S130A did not immunodeplete cyclin B1 after immunoprecipitation with anti-HA antibodies in nocodazole-treated cells. (G) Transfection of p21HA-T98A, S99A mutant, or p21HA-S130A and immunoprecipitation with anti-HA antibody showed in association with cyclin B1. (H) Transient transfection of p21HA-Wt or p21HA-T57A mutant constructs in U20S cells for 24 h and further treatment with nocodazole for 18 h followed by immunodepletion with anti-HA antibodies and immunoblotting with anti-CDK2 antibody showed CDK2 protein association. (I) p21HA-Wt or p21HA-T57A transient transfection in U20S cells treated with nocodazole for 18 h and immunodepleted with anti-HA antibody showed Cdc2 on the immunoblot. (J) p21HA-Wt or p21HA-T57A transfected in U20S cells and treatment with nocodazole for 18 h followed by immunodepletion with anti-HA antibodies and immunoblotted with cyclin A showed cyclin A association.
FIG. 7.
Phosphorylated p21 interacts specifically with serine 126 of cyclin B1. HA-tagged wild-type cyclin B1 constructs were transfected into U2OS cells for 24 h, and the cells were treated with nocodazole for another 18 h. Cells were then immunodepleted with HA monoclonal antibody and immunoblotted with p21 monoclonal antibody. Binding with hyperphosphorylated p21 protein was observed as with extracts immunodepleted with cyclin B1 antibody (Fig. 5A). Transfection of the HA-tagged cyclin B1 S126A S128A S133A S147A multiple serine-to-alanine substitution mutant very poorly bound with the phosphorylated p21, as did the S126A S128A mutant. There was no change in interaction with p21 with either the S126E S128E S133E S147E multiple serine-to-glutamic acid mutant or the S133A S147A, the S133E S147E, or the S126E S128E mutants. Phosphorylated p21 interacted very poorly with the cyclin B1 S126A mutant. However, the S128A, S133A, or S147A mutant construct interacted as normally as wild-type cyclin B1.
FIG. 8.
Kinase activity in DLD1 p21−/− cells after introduction of wild-type but not T57A-mutated p21 or in U2OS cells after introduction of wild-type but not S126A-mutated cyclin B1. (A) DLD1 p21−/− cells were transfected with a HA-tagged p21-Wt plasmid and treated with nocodazole for another 18 h. Histone H1 kinase assays were performed on the HA antibody-immunodepleted lysates, which showed H1 histone phosphorylation as early as after 3 h of nocodazole treatment. See panel B for an essential negative control. (B) p21HA-Wt or p21HA-T57A mutant plasmids were transfected in DLD1 p21−/− cells for 24 h, and the cells were treated with nocodazole for 12 h. Cell lysates were immunodepleted with anti-cyclin B1 antibodies, and H1 histone kinase assays were performed. The p21Wt construct-transfected DLD1 cells showed significantly higher phosphorylation of histone H1 compared to the T57A mutant. In nocodazole-treated DLD1−/− cells not transfected by a p21 expression plasmid, there was virtually no detectable cyclin B1-associated kinase activity (see Fig. 4A). (C) Plasmids expressing wild-type or S126A-mutated cyclin B1 tagged with HA were transfected into U2OS cells for 24 h, and the cells were treated with nocodazole for another 18 h. Cell lysates were immunoprecipitated with anti-HA antibody, and histone H1 kinase activity was determined. (D) U2OS cells were transfected with wild-type or S126A-mutated cyclin B1 tagged with HA for 24 h, and the cells were treated with nocodazole for another 18 h. Cell lysates were immunoprecipitated with anti-HA antibody and immunoblotted with anti-Cdc2 antibody.
FIG. 9.
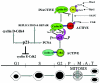
Proposed model depicting the hyperphosphorylation of p21 by active cyclin A-CDK2 at G2 phase and interaction of phosphorylated p21 with cyclin B1 at G2/M phase to promote Cdc2 kinase activity. This model predicts a dual nature for p21 function depending on cell cycle phase. The novel finding here is that hyperphosphorylated p21 possesses a unique function in G2/M phase. In the G1 phase, in the absence of DNA damage, p21 serves to facilitate assembly of the cyclin D-CDK4 complex and promotes its kinase activity and G1-phase progression. However, during the G1 phase, p53-dependent p21 induction following cellular stress results in G1 cell cycle arrest through inhibition of both CDK4 and CDK2 kinases. In G2/M, hyperphosphorylation of p21 by cyclin A-CDK2 facilitates p21 binding to S126-phosphorylated cyclin B1 and assembly of the cyclin B1-Cdc2 kinase, thereby promoting kinase activation and G2/M progression. S126-phosphorylated cyclin B1 binds to T57-phosphorylated p21. Phosphorylated p21 binds to cyclin B1 when Cdc2 is phosphorylated on Y15 and associates poorly with the complex. Dephosphorylation on Y15 by Cdc25C and phosphorylation on T161 by CAK promotes Cdc2 binding to the p21-cyclin B1 complex that becomes activated as a kinase. Thus, hyperphosphorylated p21 assembles and activates the Cdc2 kinase in the G2/M transition. Cell cycle phases are shown at the bottom. P, M, A, and T refer to prophase, metaphase, anaphase, and telophase, respectively.
Similar articles
- Transient suppression of nuclear Cdc2 activity in response to ionizing radiation.
Kim MJ, Lee JY, Lee SJ. Kim MJ, et al. Oncol Rep. 2008 May;19(5):1323-9. Oncol Rep. 2008. PMID: 18425394 - p53 regulates Cdc2 independently of inhibitory phosphorylation to reinforce radiation-induced G2 arrest in human cells.
Winters ZE, Ongkeko WM, Harris AL, Norbury CJ. Winters ZE, et al. Oncogene. 1998 Aug 13;17(6):673-84. doi: 10.1038/sj.onc.1201991. Oncogene. 1998. PMID: 9715268 - Regulation of the G2/M transition by p53.
Taylor WR, Stark GR. Taylor WR, et al. Oncogene. 2001 Apr 5;20(15):1803-15. doi: 10.1038/sj.onc.1204252. Oncogene. 2001. PMID: 11313928 Review. - Translational control during mitosis.
Le Breton M, Cormier P, Bellé R, Mulner-Lorillon O, Morales J. Le Breton M, et al. Biochimie. 2005 Sep-Oct;87(9-10):805-11. doi: 10.1016/j.biochi.2005.04.014. Biochimie. 2005. PMID: 15951098 Review.
Cited by
- Anticancer Study of a Novel Pan-HDAC Inhibitor MPT0G236 in Colorectal Cancer Cells.
Tsai FL, Huang HL, Lai MJ, Liou JP, Pan SL, Yang CR. Tsai FL, et al. Int J Mol Sci. 2023 Aug 9;24(16):12588. doi: 10.3390/ijms241612588. Int J Mol Sci. 2023. PMID: 37628767 Free PMC article. - CDDO-imidazolide induces DNA damage, G2/M arrest and apoptosis in BRCA1-mutated breast cancer cells.
Kim EH, Deng CX, Sporn MB, Liby KT. Kim EH, et al. Cancer Prev Res (Phila). 2011 Mar;4(3):425-34. doi: 10.1158/1940-6207.CAPR-10-0153. Cancer Prev Res (Phila). 2011. PMID: 21372041 Free PMC article. - p21 overexpression sensitizes osteosarcoma U2OS cells to cisplatin via evoking caspase-3 and Bax/Bcl-2 cascade.
Ding Y, Wang Y, Chen J, Hu Y, Cao Z, Ren P, Zhang Y. Ding Y, et al. Tumour Biol. 2014 Apr;35(4):3119-23. doi: 10.1007/s13277-013-1404-9. Epub 2013 Dec 11. Tumour Biol. 2014. PMID: 24323562 - Suppressor of cytokine signaling 1-dependent regulation of the expression and oncogenic functions of p21(CIP1/WAF1) in the liver.
Yeganeh M, Gui Y, Kandhi R, Bobbala D, Tobelaim WS, Saucier C, Yoshimura A, Ferbeyre G, Ramanathan S, Ilangumaran S. Yeganeh M, et al. Oncogene. 2016 Aug 11;35(32):4200-11. doi: 10.1038/onc.2015.485. Epub 2016 Jan 4. Oncogene. 2016. PMID: 26725321 - Chemoprotective effect of baicalin against cyclophosphamide induced ovarian toxicity in mice via inhibition of TGF-β.
Li X, Yue J, Kumar Y, Ma Y. Li X, et al. Heliyon. 2023 Nov 16;9(12):e22079. doi: 10.1016/j.heliyon.2023.e22079. eCollection 2023 Dec. Heliyon. 2023. PMID: 38094052 Free PMC article.
References
- Agami, R., and R. Bernards. 2000. Distinct initiation and maintenance mechanisms cooperate to induce G1 cell cycle arrest in response to DNA damage. Cell 102:55-66. - PubMed
- Akiyama, T., T. Yoshida, T. Tsujita, M. Shimizu, T. Mizukami, M. Okabe, and S. Akinaga. 1997. G1 phase accumulation induced by UCN-01 is associated with dephosphorylation of Rb and CDK2 proteins as well as induction of CDK inhibitor p21/Cip1/Waf1/Sdi1 in p53-mutated human epidermoid carcinoma A431 cells. Cancer Res. 57:1495-1501. - PubMed
- Brugarolas, J., C. Chandrasekaran, J. I. Gordon, D. Beach, T. Jacks, and G. J. Hannon. 1995. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 377:552-557. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous