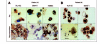Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma - PubMed (original) (raw)
Clinical Trial
. 2005 Apr 1;23(10):2346-57.
doi: 10.1200/JCO.2005.00.240.
John R Wunderlich, James C Yang, Richard M Sherry, Suzanne L Topalian, Nicholas P Restifo, Richard E Royal, Udai Kammula, Don E White, Sharon A Mavroukakis, Linda J Rogers, Gerald J Gracia, Stephanie A Jones, David P Mangiameli, Michelle M Pelletier, Juan Gea-Banacloche, Michael R Robinson, David M Berman, Armando C Filie, Andrea Abati, Steven A Rosenberg
Affiliations
- PMID: 15800326
- PMCID: PMC1475951
- DOI: 10.1200/JCO.2005.00.240
Clinical Trial
Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma
Mark E Dudley et al. J Clin Oncol. 2005.
Abstract
Purpose: We investigated the combination of lymphodepleting chemotherapy followed by the adoptive transfer of autologous tumor reactive lymphocytes for the treatment of patients with refractory metastatic melanoma.
Patients and methods: Thirty-five patients with metastatic melanoma, all but one with disease refractory to treatment with high-dose interleukin (IL) -2 and many with progressive disease after chemotherapy, underwent lymphodepleting conditioning with two days of cyclophosphamide (60 mg/kg) followed by five days of fludarabine (25 mg/m(2)). On the day following the final dose of fludarabine, all patients received cell infusion with autologous tumor-reactive, rapidly expanded tumor infiltrating lymphocyte cultures and high-dose IL-2 therapy.
Results: Eighteen (51%) of 35 treated patients experienced objective clinical responses including three ongoing complete responses and 15 partial responses with a mean duration of 11.5 +/- 2.2 months. Sites of regression included metastases to lung, liver, lymph nodes, brain, and cutaneous and subcutaneous tissues. Toxicities of treatment included the expected hematologic toxicities of chemotherapy including neutropenia, thrombocytopenia, and lymphopenia, the transient toxicities of high-dose IL-2 therapy, two patients who developed Pneumocystis pneumonia and one patient who developed an Epstein-Barr virus-related lymphoproliferation.
Conclusion: Lymphodepleting chemotherapy followed by the transfer of highly avid antitumor lymphocytes can mediate significant tumor regression in heavily pretreated patients with IL-2 refractory metastatic melanoma.
Figures
Fig 1
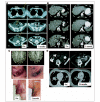
Lymphodepletion and adoptive cell transfer-induced regression of metastatic disease in diverse anatomic sites. (A) Axillary, pelvic and intraperitoneal metastases (patient 1); (B) extensive liver disease (patient 31); (C) brain and recurrent axillary metastases (patient 28); (D) multiple lung and pelvic metastases (patient 6); (E) lung and subcutaneous disease (patient 21). Pre, pretreatment.
Fig 1
Lymphodepletion and adoptive cell transfer-induced regression of metastatic disease in diverse anatomic sites. (A) Axillary, pelvic and intraperitoneal metastases (patient 1); (B) extensive liver disease (patient 31); (C) brain and recurrent axillary metastases (patient 28); (D) multiple lung and pelvic metastases (patient 6); (E) lung and subcutaneous disease (patient 21). Pre, pretreatment.
Fig 2
Loss of antigen expression in post-treatment lesions. Tumor cells in pretreatement samples expressed all antigens; however, individual antigens were lost from recurrent lesions. A) MART-1 antigen expression was lost in a recurrent lesion from patient 10. B) HLA-A2 antigen was lost in the recurrent tumor from patient 28. mθ, macrophage.
Fig 3
Granulocyte colony-stimulating factor (G-CSF) administration significantly improved the time to neutrophil recovery (two-tailed P < .03). The percentage of patients who had recovered neutrophil counts above 500/ mm3is graphed. ANC, absolute neutrophil count
Fig 4
Absolute lymphocyte count (ALC) increased transiently in some patients after adoptive cell transfer. The maximum ALC for each patient within the first 8 days after cell transfer is plotted for all nonresponding patients (NR) and objective responders (OR) who had peak ALC values above a relatively high, arbitrarily chosen value of 1,000 lymphocytes/mm3.
Fig 5
CD8+lymphocyte counts recovered more rapidly than CD4+lymphocytes. Each symbol represents an individual patient. All available data is included, but measurements were not available for all patients at each time increment. Open symbols represent five patients who were treated with a prior course of lymphodepleting chemotherapy. Horizontal lines indicate normal limits. A) Recovery of CD4+cells. B) Recovery of CD8+cells. Bars represent mean values of all patients.
Fig 6
Transferred cells persisted for extended time in responding patients. Each tumor-infiltrating lymphocyte administered on day 0 contained a tumor reactive clone with the indicated T-cell receptor beta chain variable region (Vβ) expression or HLA-A2/MART-1-26-35 (27L) tetrameter (A2/MART-1)-binding. The percentage of CD8+ cells recognized by the indicated reagent is indicated. Pre, pretreatment peripheral blood lymphocytes (PBL). Post, PBL on the day indicated after cell transfer.
Similar articles
- Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens.
Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA. Dudley ME, et al. J Clin Oncol. 2008 Nov 10;26(32):5233-9. doi: 10.1200/JCO.2008.16.5449. Epub 2008 Sep 22. J Clin Oncol. 2008. PMID: 18809613 Free PMC article. Clinical Trial. - Phase II clinical trial of adoptive cell therapy for patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and low-dose interleukin-2.
Nguyen LT, Saibil SD, Sotov V, Le MX, Khoja L, Ghazarian D, Bonilla L, Majeed H, Hogg D, Joshua AM, Crump M, Franke N, Spreafico A, Hansen A, Al-Habeeb A, Leong W, Easson A, Reedijk M, Goldstein DP, McCready D, Yasufuku K, Waddell T, Cypel M, Pierre A, Zhang B, Boross-Harmer S, Cipollone J, Nelles M, Scheid E, Fyrsta M, Lo CS, Nie J, Yam JY, Yen PH, Gray D, Motta V, Elford AR, DeLuca S, Wang L, Effendi S, Ellenchery R, Hirano N, Ohashi PS, Butler MO. Nguyen LT, et al. Cancer Immunol Immunother. 2019 May;68(5):773-785. doi: 10.1007/s00262-019-02307-x. Epub 2019 Feb 11. Cancer Immunol Immunother. 2019. PMID: 30747243 Free PMC article. Clinical Trial. - Treatment of metastatic uveal melanoma with adoptive transfer of tumour-infiltrating lymphocytes: a single-centre, two-stage, single-arm, phase 2 study.
Chandran SS, Somerville RPT, Yang JC, Sherry RM, Klebanoff CA, Goff SL, Wunderlich JR, Danforth DN, Zlott D, Paria BC, Sabesan AC, Srivastava AK, Xi L, Pham TH, Raffeld M, White DE, Toomey MA, Rosenberg SA, Kammula US. Chandran SS, et al. Lancet Oncol. 2017 Jun;18(6):792-802. doi: 10.1016/S1470-2045(17)30251-6. Epub 2017 Apr 7. Lancet Oncol. 2017. PMID: 28395880 Free PMC article. Clinical Trial. - Adoptive cell transfer therapy.
Dudley ME, Rosenberg SA. Dudley ME, et al. Semin Oncol. 2007 Dec;34(6):524-31. doi: 10.1053/j.seminoncol.2007.09.002. Semin Oncol. 2007. PMID: 18083376 Free PMC article. Review. - Active immunotherapy of metastatic melanoma with allogeneic melanoma lysates and interferon alpha.
Vaishampayan U, Abrams J, Darrah D, Jones V, Mitchell MS. Vaishampayan U, et al. Clin Cancer Res. 2002 Dec;8(12):3696-701. Clin Cancer Res. 2002. PMID: 12473578 Review.
Cited by
- Natural killer cell-based cancer immunotherapy: from basics to clinical trials.
Shi Y, Hao D, Qian H, Tao Z. Shi Y, et al. Exp Hematol Oncol. 2024 Oct 16;13(1):101. doi: 10.1186/s40164-024-00561-z. Exp Hematol Oncol. 2024. PMID: 39415291 Free PMC article. Review. - Syngeneic Mouse Models for Pre-Clinical Evaluation of CAR T Cells.
Ahmed EN, Cutmore LC, Marshall JF. Ahmed EN, et al. Cancers (Basel). 2024 Sep 18;16(18):3186. doi: 10.3390/cancers16183186. Cancers (Basel). 2024. PMID: 39335157 Free PMC article. Review. - Immune responses and immunotherapeutic approaches in the treatment against cancer.
Leong SP. Leong SP. Clin Exp Metastasis. 2024 Aug;41(4):473-493. doi: 10.1007/s10585-024-10300-7. Epub 2024 Aug 18. Clin Exp Metastasis. 2024. PMID: 39155358 Free PMC article. Review. - Enhancing natural killer cells proliferation and cytotoxicity using imidazole-based lipid nanoparticles encapsulating interleukin-2 mRNA.
Delehedde C, Ciganek I, Bernard PL, Laroui N, Da Silva CC, Gonçalves C, Nunes J, Bennaceur-Griscelli AL, Imeri J, Huyghe M, Even L, Midoux P, Rameix N, Guittard G, Pichon C. Delehedde C, et al. Mol Ther Nucleic Acids. 2024 Jun 26;35(3):102263. doi: 10.1016/j.omtn.2024.102263. eCollection 2024 Sep 10. Mol Ther Nucleic Acids. 2024. PMID: 39104868 Free PMC article. - Advancing non-small cell lung cancer treatment: the power of combination immunotherapies.
Wu Y, Yu G, Jin K, Qian J. Wu Y, et al. Front Immunol. 2024 Jul 2;15:1349502. doi: 10.3389/fimmu.2024.1349502. eCollection 2024. Front Immunol. 2024. PMID: 39015563 Free PMC article. Review.
References
- Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–1321. - PubMed
- Cheever MA, Greenberg PD, Fefer A. Specificity of adoptive chemoimmunotherapy of established syngeneic tumors. J Immunol. 1980;125:711–714. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical