A role for prefrontal calcium-sensitive protein phosphatase and kinase activities in working memory - PubMed (original) (raw)
Comparative Study
. 2005 Mar-Apr;12(2):103-10.
doi: 10.1101/lm.89405.
Affiliations
- PMID: 15805309
- PMCID: PMC1074327
- DOI: 10.1101/lm.89405
Comparative Study
A role for prefrontal calcium-sensitive protein phosphatase and kinase activities in working memory
Jason D Runyan et al. Learn Mem. 2005 Mar-Apr.
Abstract
The prefrontal cortex is involved in the integration and interpretation of information for directing thoughts and planning action. Working memory is defined as the active maintenance of information in mind and is thought to lie at the core of many prefrontal functions. Although dopamine and other neurotransmitters have been implicated, the intracellular events activated by their receptors that influence working memory are poorly understood. We demonstrate that working memory involves transient changes in prefrontal G(q/11)-signaling and in calcium-dependent intracellular protein phosphatase and kinase activity. Interestingly, inhibition of the calcium activated phosphatase calcineurin impaired, while calcium/calmodulin dependent kinase II (CaMKII) and calcium-dependent protein kinase C (PKC) enhanced, working memory. Our findings suggest that the active maintenance of information required for working memory involves transient changes in the balance of these enzymes' activities.
Figures
Figure 1.
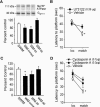
mPFC activity and D1 receptors are required for performance in a delay match-to-place working memory task. Average latency to hidden platform for location and match trials for animals infused into the mPFC with the following: (A) muscimol (0.5 μg), a GABA agonist; (B) SCH-23390 (1 μg), a D1/D5 receptor antagonist; (C) SKF-38393 (1 μg), a D1/D5 receptor agonist; and (D) SKF-38393 (0.08 μg) compared with their respective vehicle infused controls. (E) Working memory performance follows an inverted-U response curve to D1 receptor stimulation (expressed as the delta [Δ] latency between the location and match trials). (F) Average latency to hidden platform for location and match trials for animals infused into the hippocampus with SCH-23390 (1 μg). *P < 0.05 comparing location to match trials; †P < 0.05 comparing match trials between groups.
Figure 2.
Spatial working memory involves PLC and calcineurin activities. (A) Representative Gq/11α and Na+/K+ ATPase Western blots performed on mPFC membrane extracts taken at time-points during the delay match-to-place task compared against swim controls. Summary results showing average Gq/11α immunoreactivity as a percentage of that obtained for swim controls. *P < 0.05. (B) Average latency to hidden platform for location trials and match trials during the delay match-to-place task for intra-mPFC infused vehicle and U73122 (0.26 μg). *P < 0.05 comparing location to match trials; †P < 0.05 comparing match trials between groups. (C) mPFC calcineurin activity expressed as percentage swim control at time-points during the delay match-to-place working memory task. *P < 0.05. (D) Average latency to hidden platform for location and match trials during the delay match-to-place task for intra-mPFC vehicle, and cyclosporin A (0.3 μg or 0.7 μg) animals. *P < 0.05 comparing location to match trials.
Figure 3.
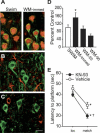
CaMKII activity negatively influences spatial working memory. (A) Representative confocal images of phospho-CaMKII (green) and NeuN (red) immunoreactivity of mPFC tissue sections taken from animals performing the working memory task (immediate) or from swim control. Phospho-CaMKII immunoreactivity was not detected in GFAP-expressing astrocytes (red) (B) or GAD67-expressing inhibitory neurons (C). Scale bar for all images = 10 μm. (D) mPFC CaMKII activity expressed as percent swim control at time-points during the delay match-to-place working memory task. (E) Average latency to hidden platform for location and match trials for intra-mPFC KN-93–infused (1 μg), a CaMKII inhibitor, or vehicle-infused animals. *P < 0.05 comparing location to match trials; †P < 0.05 comparing match trials between groups.
Figure 4.
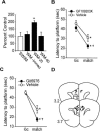
PKC activity negatively influences performance in the delay match-to-place task. (A) PKC activity expressed as percent swim control at time-points during the working memory task. Average latency to hidden platform for location and match trials for intra-mPFC. (B) GF109203X (1 μg), a PKC inhibitor, and (C) Gö6976-infused (1 μg), a Ca2+-dependent PKC inhibitor, animals compared with vehicle controls. (D) Illustration of coronal sections (3.2 mm and 3.7 mm to bregma) of the mPFC indicating the non-redundant infusion sites for the animals in this study. *P < 0.05 comparing location to match trials; †P < 0.05 comparing match trials between groups.
Similar articles
- Molecular activity underlying working memory.
Dash PK, Moore AN, Kobori N, Runyan JD. Dash PK, et al. Learn Mem. 2007 Aug 9;14(8):554-63. doi: 10.1101/lm.558707. Print 2007 Aug. Learn Mem. 2007. PMID: 17690339 Review. - Prefrontal inositol triphosphate is molecular correlate of working memory in nonhuman primates.
López-Téllez JF, López-Aranda MF, Navarro-Lobato I, Masmudi-Martín M, Montañez EM, Calvo EB, Khan ZU. López-Téllez JF, et al. J Neurosci. 2010 Feb 24;30(8):3067-71. doi: 10.1523/JNEUROSCI.4565-09.2010. J Neurosci. 2010. PMID: 20181603 Free PMC article. - Bidirectional regulation of Ca2+/calmodulin-dependent protein kinase II activity by dopamine D4 receptors in prefrontal cortex.
Gu Z, Yan Z. Gu Z, et al. Mol Pharmacol. 2004 Oct;66(4):948-55. doi: 10.1124/mol.104.001404. Epub 2004 Jun 30. Mol Pharmacol. 2004. PMID: 15229297 - Molecular mechanisms of working memory.
Khan ZU, Muly EC. Khan ZU, et al. Behav Brain Res. 2011 Jun 1;219(2):329-41. doi: 10.1016/j.bbr.2010.12.039. Epub 2011 Jan 11. Behav Brain Res. 2011. PMID: 21232555 Review. - Aberrant CaMKII activity in the medial prefrontal cortex is associated with cognitive dysfunction in ADHD model rats.
Yabuki Y, Shioda N, Maeda T, Hiraide S, Togashi H, Fukunaga K. Yabuki Y, et al. Brain Res. 2014 Apr 4;1557:90-100. doi: 10.1016/j.brainres.2014.02.025. Epub 2014 Feb 19. Brain Res. 2014. PMID: 24561222
Cited by
- Distinct prefrontal molecular mechanisms for information storage lasting seconds versus minutes.
Runyan JD, Dash PK. Runyan JD, et al. Learn Mem. 2005 May-Jun;12(3):232-8. doi: 10.1101/lm.92405. Learn Mem. 2005. PMID: 15930501 Free PMC article. - Lead dysregulates serine/threonine protein phosphatases in human neurons.
Rahman A, Brew BJ, Guillemin GJ. Rahman A, et al. Neurochem Res. 2011 Feb;36(2):195-204. doi: 10.1007/s11064-010-0300-6. Epub 2010 Nov 4. Neurochem Res. 2011. PMID: 21046238 Free PMC article. - Noradrenergic Suppression of Persistent Firing in Hippocampal CA1 Pyramidal Cells through cAMP-PKA Pathway.
Valero-Aracama MJ, Reboreda A, Arboit A, Sauvage M, Yoshida M. Valero-Aracama MJ, et al. eNeuro. 2021 Mar 22;8(2):ENEURO.0440-20.2020. doi: 10.1523/ENEURO.0440-20.2020. Print 2021 Mar-Apr. eNeuro. 2021. PMID: 33637539 Free PMC article. - Altered regulation of protein kinase a activity in the medial prefrontal cortex of normal and brain-injured animals actively engaged in a working memory task.
Kobori N, Moore AN, Dash PK. Kobori N, et al. J Neurotrauma. 2015 Jan 15;32(2):139-48. doi: 10.1089/neu.2014.3487. Epub 2014 Nov 13. J Neurotrauma. 2015. PMID: 25027811 Free PMC article. - PERK Regulates Working Memory and Protein Synthesis-Dependent Memory Flexibility.
Zhu S, Henninger K, McGrath BC, Cavener DR. Zhu S, et al. PLoS One. 2016 Sep 14;11(9):e0162766. doi: 10.1371/journal.pone.0162766. eCollection 2016. PLoS One. 2016. PMID: 27627766 Free PMC article.
References
- Arnsten, A.F., Cai, J.X., Murphy, B.L., and Goldman-Rakic, P.S. 1994. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology 116: 143-151. - PubMed
- Arthur, J.M., Collinsworth, G.P., Gettys, T.W., and Raymond, J.R. 1999. Agonist-induced translocation of Gq/11α immunoreactivity directly from plasma membrane in MDCK cells. Am. J. Physiol. 276: F528-F534. - PubMed
- Baddeley, A. 1992. Working memory. Science 255: 556-559. - PubMed
- Baeg, E.H., Kim, Y.B., Huh, K., Mook-Jung, I., Kim, H.T., and Jung, M.W. 2003. Dynamics of population code for working memory in the prefrontal cortex. Neuron 40: 177-188. - PubMed
- Birnbaum, S.G., Yuan, P.X., Wang, M., Vijayraghavan, S., Bloom, A.K., Davis, D.J., Gobeske, K.T., Sweatt, J.D., Manji, H.K., and Arnsten, A.F. 2004. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science 306: 882-884. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources