Kissing complex RNAs mediate interaction between the Fragile-X mental retardation protein KH2 domain and brain polyribosomes - PubMed (original) (raw)
Comparative Study
. 2005 Apr 15;19(8):903-18.
doi: 10.1101/gad.1276805. Epub 2005 Apr 1.
Affiliations
- PMID: 15805463
- PMCID: PMC1080130
- DOI: 10.1101/gad.1276805
Comparative Study
Kissing complex RNAs mediate interaction between the Fragile-X mental retardation protein KH2 domain and brain polyribosomes
Jennifer C Darnell et al. Genes Dev. 2005.
Abstract
Fragile-X mental retardation is caused by loss of function of a single gene encoding the Fragile-X mental retardation protein, FMRP, an RNA-binding protein that harbors two KH-type and one RGG-type RNA-binding domains. Previous studies identified intramolecular G-quartet RNAs as high-affinity targets for the RGG box, but the relationship of RNA binding to FMRP function and mental retardation remains unclear. One severely affected patient harbors a missense mutation (I304N) within the second KH domain (KH2), and some evidence suggests this domain may be involved in the proposed role of FMRP in translational regulation. We now identify the RNA target for the KH2 domain as a sequence-specific element within a complex tertiary structure termed the FMRP kissing complex. We demonstrate that the association of FMRP with brain polyribosomes is abrogated by competition with the FMRP kissing complex RNA, but not by high-affinity G-quartet RNAs. We conclude that mental retardation associated with the I304N mutation, and likely the Fragile-X syndrome more generally, may relate to a crucial role for RNAs harboring the kissing complex motif as targets for FMRP translational regulation.
Figures
Figure 1.
Selection of FMRP KH2 RNA ligands. (A) Filter binding assays using full-length wild-type (Wt) or I304N mutant FMRP bound to the total RNA pool present after eight rounds of selection. (B) Gel-shift assays of T7-tagged FMRP KH1/2 protein with kc2 RNA, performed in the presence of indicated antibodies. Two conformations of free kc2 RNA are evident in lanes in which the RNA has not shifted, and only the faster migrating form shifts and supershifts in the presence of T7 antibody. (C) Filter binding assays comparing binding of KH1/2 and the isolated KH2 domain to kc2 RNA. (D) Filter binding assays comparing the ability of KH2 to bind isolated kc1 RNA and the kc1 RNA inserted into the middle of the 5′ UTR of luciferase mRNA.
Figure 2.
Magnesium-dependent binding suggests a loop-loop interaction for the KH2 RNA ligands. (A) FMRP-kc1 binding is dependent on Mg+2. Filter binding assays were performed in the presence of Mg+2 or EDTA, as indicated, to kc1, or, as a control, to sc1 (RGG-box RNA ligand) with full-length FMRP. (B) FMRP-kc1 binding as a function of Mg+2 concentration. Analysis of kc1 binding curves to KH1/2 at indicated Mg+2 concentrations is shown; the slope is 0.94, suggesting one specifically bound Mg+2 ion participates in the RNA:protein interaction. (C) Lead-acetate cleavage of kc1 RNA in the presence of competing MgCl2. Cleavage sites (arrows labeled 1-3) that are effectively competed by increasing Mg+2 concentrations (0, 10, 25, 100, 250 mM Mg+2; lanes _4_-8) indicate likely Mg+2-binding sites. Nonspecific cleavage corresponding to single-stranded “hinge” is indicated by the bracket. RNAs treated with alkaline hydrolysis (AH; lane 1) and RNase T1 (lane 2) or untreated RNA (lane 3) are shown. (D) mfold 3.1 structures of kc1, kc2, and Δkc2 RNAs, with sites of lead-acetate cleavage indicated (arrows). Δkc2 was constructed by deleting bracketed areas shown in kc2.
Figure 3.
Chemical modification of KH2 RNA ligand kc1. Native (“N”) or semidenatured (EDTA; “E”) RNA was treated for the indicated times with DMS (which modifies single-stranded [non-Watson-Crick-paired] A > C residues), CMCT (which modifies single-stranded U > G), or kethoxal (which modifies single-stranded G), or untreated (first lane) to control for natural stops in primer extension. Several residues protected from modification in the native structure (suggesting formation of new Watson-Crick base pairs in a Mg+2-dependent manner) are illustrated with bullets; the hinge region, which is always modified, and a stable stem, which is always protected from modification, are indicated. Several nucleotides with potential to form Watson-Crick base pairs (A4, A10, U29, and U52) are also indicated with arrows and discussed in the next section (Fig. 4). Numbers refer to positions in Δkc2, Table 1, and Figures 2 and 4B.
Figure 4.
Compensatory mutation analysis of Watson-Crick interactions in selected KH2 RNA ligands. (A,B) Diagram of kc1 (A) or Δkc2 (B) drawn to incorporate loop-loop interactions suggested by chemical probing. (B) Nucleotide numbering refers to Δkc2. (C) Mutations made in Δkc2 to test the presence of duplex RNA interactions (“yellow” stem) suggested by chemical probing, which would result in a loop-loop pseudoknot. Indicated mutations (A10 to G10; “A10G”, etc.) were tested for KH2 binding by filter binding assay. Binding is indicated relative to wild-type RNA (++++) to essentially non-detectable specific binding (-). For examples, see E and Supplementary Figure S3. A base pair is indicated by abrogation of binding by mutation of either the 5′ (5′ mut) or 3′ (3′ mut) nucleotide, and restoration of binding with compensatory mutations (dbl). (D) Compensatory mutation analysis to test a second potential interaction between the loops in Δkc2 (“purple” base pair), tested as in C.(E) Illustrative filter binding curves corresponding to data in D. Filter binding curves for the data in C are shown in Supplementary Figure S3. (F) Compensatory mutation analysis of a Mg+2-dependent stem (“green” stem) suggested by mfold and chemical probing in Δkc2, tested as in C.
Figure 5.
Mutational analysis of the non-Watson-Crick-paired regions of Δkc2. (A) Indicated nucleotides and substituted mutations for each were assessed for binding to KH2 by filter binding assay. Results are summarized as a comparison to wild-type Δkc2 Kd (80 nM) and grouped by descending Kd. Untested mutations were those in which substitution of the indicated nucleotide at that position resulted in global misfolding of Δkc2 as predicted by mfold 3.1. Phylogeny refers to the number of occurrences of a particular nucleotide out of 13 different sequences (kc1-kc13). NA (not applicable) means those nucleotides are from the fixed regions of the selected clones and are invariant. Asterisks denote the G19-G46 pair that covaries with one A19-A46 base pair. (B) Δkc2 RNA ligand redrawn to focus on loop sequences. Probable non-Watson-Crick-paired nucleotides (tested in A) are shown in red, Watson-Crick interacting nucleotides are shown in black, and 5′ and 3′ stems are shown as anti-parallel arrows. Nucleotides in which mutational analysis showed essentially no tolerance for mutation and phylogenetic variation are indicated by red arrowheads, those with some tolerance for mutation and phylogenetic variation are indicated by yellow arrowheads, and those that are most permissive for mutation and phylogenetic variation are indicated by green arrowheads. (C) Consensus of Δkc2 RNA KH2 ligand based upon experimental data. Red nucleotides are those most likely to be involved in KH2 binding. K is a G or U, R is an A or G, W is an A or U, N can be any nucleotide, and S refers to a Watson-Crick base pair.
Figure 6.
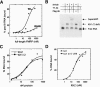
kc2 RNA competes FMRP off of mouse brain polyribosomes in a dose-dependent manner. (A) P8 mouse cerebral cortical extracts were separated by sucrose density gradient (20%-50%) centrifugation; positions of the 80S monosome and polyribosomes are indicated. Indicated amounts of kc2 RNA were added to brain lysates and incubated for 15 min at room temperature prior to centrifugation. A254 profile of sucrose density gradients for the lowest and highest kc2 RNA concentrations are shown; A254 profiles for all kc2 concentrations were indistinguishable (data not shown). (B) Western blots of indicated fractions from sucrose density gradients incubated with the indicated kc2 concentration were probed with the FMRP monoclonal antibody 1C3. Fractions corresponding to 40S, 60S, and 80S ribosomal peaks are indicated. Ribosomal protein S6 was unshifted at all kc2 concentrations (data not shown). (C) Quantitation by chemiluminescence of the Western blot data in B (Versadoc imaging); the percentage of FMRP present in each fraction as a function of the total FMRP signal in that gradient is indicated. Inset shows a plot of the percent of FMRP shifted off the polyribosomes plotted against kc2 concentration (nanomolar). The half-maximal concentration of kc2 able to compete FMRP off of polysome fractions is ∼100 nM kc2 RNA.
Figure 7.
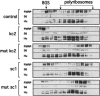
kc2 RNA competes FMRP off polyribosomes. Western blot as in Figure 6 of sucrose density gradients in which indicated RNAs were used to compete FMRP polyribosomal association; mut kc2 harbors a single point mutation in kc2 RNA (G20 to C20; see Fig. 4E for binding curve), sc1 is a high-affinity G-quartet ligand for FMRP RGG box, and mut sc1 harbors a single point mutation that destroys G-quartet formation and FMRP binding. Western blots were probed with antibodies to the indicated proteins, including FMRP, ribosomal protein S6, and the Hu family of RNA-binding proteins.
Figure 8.
Specificity of FMRP interaction with polyribosomal targets. A254 traces (A) and Western blot analysis for FMRP or ribosomal S6 protein (B), as in Figure 6. No RNA was added to the control lysates (control). BC1 RNA (produced by in vitro transcription and gel purified) and yeast tRNA at the indicated concentrations fail to compete with FMRP binding to polyribosomes.
Similar articles
- Discrimination of common and unique RNA-binding activities among Fragile X mental retardation protein paralogs.
Darnell JC, Fraser CE, Mostovetsky O, Darnell RB. Darnell JC, et al. Hum Mol Genet. 2009 Sep 1;18(17):3164-77. doi: 10.1093/hmg/ddp255. Epub 2009 Jun 1. Hum Mol Genet. 2009. PMID: 19487368 Free PMC article. - RNAs that interact with the fragile X syndrome RNA binding protein FMRP.
Sung YJ, Conti J, Currie JR, Brown WT, Denman RB. Sung YJ, et al. Biochem Biophys Res Commun. 2000 Sep 7;275(3):973-80. doi: 10.1006/bbrc.2000.3405. Biochem Biophys Res Commun. 2000. PMID: 10973830 - The C terminus of fragile X mental retardation protein interacts with the multi-domain Ran-binding protein in the microtubule-organising centre.
Menon RP, Gibson TJ, Pastore A. Menon RP, et al. J Mol Biol. 2004 Oct 8;343(1):43-53. doi: 10.1016/j.jmb.2004.08.024. J Mol Biol. 2004. PMID: 15381419 - FMRP RNA targets: identification and validation.
Darnell JC, Mostovetsky O, Darnell RB. Darnell JC, et al. Genes Brain Behav. 2005 Aug;4(6):341-9. doi: 10.1111/j.1601-183X.2005.00144.x. Genes Brain Behav. 2005. PMID: 16098133 Review. - The fragile X mental retardation protein, FMRP, recognizes G-quartets.
Darnell JC, Warren ST, Darnell RB. Darnell JC, et al. Ment Retard Dev Disabil Res Rev. 2004;10(1):49-52. doi: 10.1002/mrdd.20008. Ment Retard Dev Disabil Res Rev. 2004. PMID: 14994288 Review.
Cited by
- FMRP cooperates with miRISC components to repress translation and regulate neurite morphogenesis in Drosophila.
Kaul N, Pradhan SJ, Boin NG, Mason MM, Rosales J, Starke EL, Wilkinson EC, Chapman EG, Barbee SA. Kaul N, et al. RNA Biol. 2024 Jan;21(1):11-22. doi: 10.1080/15476286.2024.2392304. Epub 2024 Aug 27. RNA Biol. 2024. PMID: 39190491 Free PMC article. - The RNA-Binding Function of Ribosomal Proteins and Ribosome Biogenesis Factors in Human Health and Disease.
Catalanotto C, Barbato C, Cogoni C, Benelli D. Catalanotto C, et al. Biomedicines. 2023 Nov 4;11(11):2969. doi: 10.3390/biomedicines11112969. Biomedicines. 2023. PMID: 38001969 Free PMC article. Review. - FMRP phosphorylation and interactions with Cdh1 regulate association with dendritic RNA granules and MEF2-triggered synapse elimination.
Wilkerson JR, Ifrim MF, Valdez-Sinon AN, Hahn P, Bowles JE, Molinaro G, Janusz-Kaminska A, Bassell GJ, Huber KM. Wilkerson JR, et al. Neurobiol Dis. 2023 Jun 15;182:106136. doi: 10.1016/j.nbd.2023.106136. Epub 2023 Apr 28. Neurobiol Dis. 2023. PMID: 37120096 Free PMC article. - FMRP binds Per1 mRNA and downregulates its protein expression in mice.
Tang X, Zhang J, Li X, Hu Y, Liu D, Li JD, Lu R. Tang X, et al. Mol Brain. 2023 Apr 5;16(1):33. doi: 10.1186/s13041-023-01023-z. Mol Brain. 2023. PMID: 37020302 Free PMC article. - Function of FMRP Domains in Regulating Distinct Roles of Neuronal Protein Synthesis.
D'Souza MN, Ramakrishna S, Radhakrishna BK, Jhaveri V, Ravindran S, Yeramala L, Nair D, Palakodeti D, Muddashetty RS. D'Souza MN, et al. Mol Neurobiol. 2022 Dec;59(12):7370-7392. doi: 10.1007/s12035-022-03049-1. Epub 2022 Oct 1. Mol Neurobiol. 2022. PMID: 36181660
References
- Argaman L. and Altuvia, S. 2000. fhlA repression by OxyS RNA: Kissing complex formation at two sites results in a stable antisense-target RNA complex. J. Mol. Biol. 300: 1101-1112. - PubMed
- Bardoni B., Schenck, A., and Mandel, J.L. 1999. A novel RNA-binding nuclear protein that interacts with the fragile X mental retardation (FMR1) protein. Hum. Mol. Genet. 8: 2557-2566. - PubMed
- Bartel D.P. 2004. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 116: 281-297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS40955/NS/NINDS NIH HHS/United States
- GM07739/GM/NIGMS NIH HHS/United States
- R01 NS34389/NS/NINDS NIH HHS/United States
- R01 NS040955/NS/NINDS NIH HHS/United States
- R01 HD040647/HD/NICHD NIH HHS/United States
- R01 NS034389/NS/NINDS NIH HHS/United States
- R01 HD40647/HD/NICHD NIH HHS/United States
- R01 HG001363/HG/NHGRI NIH HHS/United States
- T32 GM007739/GM/NIGMS NIH HHS/United States
- R01 HG01363/HG/NHGRI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases