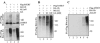Ubiquitin ligase MKRN1 modulates telomere length homeostasis through a proteolysis of hTERT - PubMed (original) (raw)
Ubiquitin ligase MKRN1 modulates telomere length homeostasis through a proteolysis of hTERT
Jun Hyun Kim et al. Genes Dev. 2005.
Abstract
Telomere homeostasis is regulated by telomerase and a collection of associated proteins. Telomerase is, in turn, regulated by post-translational modifications of the rate-limiting catalytic subunit hTERT. Here we show that disruption of Hsp90 by geldanamycin promotes efficient ubiquitination and proteasome-mediated degradation of hTERT. Furthermore, we have used the yeast two-hybrid method to identify a novel RING finger gene (MKRN1) encoding an E3 ligase that mediates ubiquitination of hTERT. Overexpression of MKRN1 in telomerase-positive cells promotes the degradation of hTERT and decreases telomerase activity and subsequently telomere length. Our data suggest that MKRN1 plays an important role in modulating telomere length homeostasis through a dynamic balance involving hTERT protein stability.
Figures
Figure 1.
hTERT is sensitive to the Hsp90 antagonist geldanamycin. (A) Down-regulation of ectopically expressed hTERT by GA. H1299 cells transfected with Flag-hTERT or Flag-TRF1 were treated with 0.1 μM GA for the indicated times. Lysates were resolved on 8% SDS-PAGE and analyzed by immunoblotting using an anti-Flag antibody probe. Cells were pretreated with 10 μM MG132 or E-64 for 2 h, either alone or before treatment with GA. (B) Concentration dependence of GA on hTERT. H1299 cells transfected with Flag-hTERT or hTERT-HA were treated for 2 h with the GA concentration indicated. The hTERT proteins were visualized with anti-Flag or anti-HA antibodies as marked by each blot. (C) Down-regulation of endogenous hTERT by GA. H1299 cells were treated with 0.1 μM GA for the indicated times, and cell lysates were analyzed by immunoprecipitation with anti-hTERT antibody followed by immunoblotting using the same antibody probe. Cells were pretreated with 10 μM MG132 for 2 h, either alone or before treatment with GA. Molecular weight markers are shown in kilodaltons. (D) H1299 cells were treated with 0.1 μM GA for the indicated times, and cell lysates were analyzed for telomerase activity by the TRAP assay. The lane labeled LB corresponds to the negative control (lysis buffer only). ITAS represents the internal telomerase assay standard. (E) Saos-2 cells transfected with Flag-hTERT or hTERT-HA or empty vector were untreated or treated with 0.1 μM GA for 16 h, and cell lysates were analyzed for telomerase activity.
Figure 2.
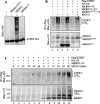
hTERT is ubiquitinated prior to proteasome-mediated degradation. (A) H1299 cells were cotransfected with Flag-hTERT and HA-ubiquitin. Cells were either untreated or treated for 2 h with 10 μg/mL ALLnN or 0.1 μM GA, or a combination of the two as specified. Immunoprecipitation was performed with anti-Flag antibody before probing with anti-hTERT or anti-HA antibodies as indicated. Molecular weight markers are shown in kilodaltons. (B) H1299 cells cotransfected with Flag-hTERT and HA-ubiquitin were treated for 1 h with the GA concentration indicated in the presence of 10 μM MG132. (C) H1299 cells cotransfected with Flag-hTERT and HA-ubiquitin were either untreated or treated for 2 h with 10 μg/mL ALLnN as specified. Proteins were immunoprecipitated with anti-Flag antibody followed by immunoblotting using an anti-HA antibody probe.
Figure 3.
MKRN1 interacts with hTERT. (A) Analysis of the physical interaction between hTERT and MKRN1 using the yeast two-hybrid assay. Five domains of hTERT were analyzed for binding to MKRN1. TRF1 bait was used as a negative control. Blue signal on the SG-HWU/X plate indicates activation of the reporter gene LacZ. (S) Synthetic; (G) galactose; (H) histidine (–); (W) tryptophan (–); (U) uracil (–); (X) X-gal. (B) Schematic diagram of MKRN1 domains. Amino acid sequences surrounding the RING finger domain in MKRN1 and mutants used in this study. Cysteines and a histidine in the RING finger domain are shown in red. The mutated residue in MKRN1H307E is shown in blue. (C) H1299 cells cotransfected with hTERT-HA and MKRN1-V5 were either untreated or treated for 2 h with 10 μg/mL ALLnN. Anti-HA immunoprecipitates were analyzed by immunoblotting using an anti-V5 antibody probe. The left panel shows the immunoblot of cell lysates (3% of the precipitated lysates) probed with anti-V5 antibody. hTERT-HA was visualized with an anti-HA antibody probe. (D) H1299 cells cotransfected with Flag-hTERT and MKRN1-V5 were either untreated or treated for 2 h with 10 μM MG132. Anti-Flag immunoprecipitates were analyzed by immunoblotting using an anti-V5 antibody probe. The left panel shows the immunoblot of cell lysates (3% of the precipitated lysates) probed with anti-V5 antibody. Flag-hTERT was visualized with an anti-Flag antibody probe.
Figure 4.
MKRN1 functions as an E3 ubiquitin-ligase for hTERT in vitro and in vivo. (A) HA-tagged hTERT (residues 946–1132) was incubated with MKRN1 or mutants in the presence of E1, E2, and His6-ubiquitin. Samples were resolved by 6% SDS-PAGE and analyzed by immunoblotting with an anti-HA antibody probe. (B) H1299 cells were cotransfected with Flag-hTERT, HA-ubiquitin, MKRN1-V5, and together with either MKRN1H307E-V5 or MKRN1ΔC-V5 as specified. Cells were untreated or treated with 10 μM MG132 for 2 h. Anti-Flag immunoprecipitates and cell lysates were analyzed by immunoblotting with anti-HA antibody and anti-V5 antibody probes, respectively. (C) H1299 cells cotransfected with Flag-hTERT and HA-ubiquitin, and together with or without MKRN1-V5 as indicated, were treated with 10 μM MG132 for the indicated times. Anti-Flag immunoprecipitates and cell lysates were analyzed by immunoblotting with anti-HA antibody and anti-V5 antibody probes, respectively.
Figure 5.
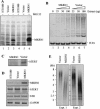
Overexpression of MKRN1 decreases telomerase activity and telomere length. (A) HT1080 cells were stably transfected with MKRN1-V5 or an empty vector. The cells were untreated or treated for 2 h with 10 μM MG132. Cell lysates were analyzed by immunoblotting with anti-V5 antibody probe. (B) Stable HT1080 cell lines (MKRN1 and vector) were harvested at 45 PD, and telomerase activities were measured with different amounts of proteins using the TRAP assay. (C) Cell lysates from stable HT1080 cell lines were analyzed by immunoprecipitation with anti-hTERT antibody followed by immunoblotting using the same antibody probe. (D) Representative results of RT–PCR analysis for the expression of MKRN1, hTERT, and hTR in stable HT1080 cell lines. RT–PCR products from each sample were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) signal. (E) Stable HT1080 cell lines were harvested at 45 PD, and genomic DNA was digested with RsaI and HinfI, followed by Southern blotting using a telomere repeat probe.
Similar articles
- Differentiation linked regulation of telomerase activity by Makorin-1.
Salvatico J, Kim JH, Chung IK, Muller MT. Salvatico J, et al. Mol Cell Biochem. 2010 Sep;342(1-2):241-50. doi: 10.1007/s11010-010-0490-x. Epub 2010 May 16. Mol Cell Biochem. 2010. PMID: 20473778 - Polo-like Kinase 1 (Plk1) Up-regulates Telomerase Activity by Affecting Human Telomerase Reverse Transcriptase (hTERT) Stability.
Huang Y, Sun L, Liu N, Wei Q, Jiang L, Tong X, Ye X. Huang Y, et al. J Biol Chem. 2015 Jul 24;290(30):18865-73. doi: 10.1074/jbc.M114.635375. Epub 2015 Jun 12. J Biol Chem. 2015. PMID: 26070557 Free PMC article. - [Molecular mechanisms involved in the regulation of telomerase activity].
Liu WJ, Ding J. Liu WJ, et al. Sheng Li Ke Xue Jin Zhan. 2001 Jul;32(3):220-4. Sheng Li Ke Xue Jin Zhan. 2001. PMID: 12545793 Review. Chinese. - [Progress on telomere and telomerase].
Yang X, Tao M. Yang X, et al. Wei Sheng Yan Jiu. 2004 May;33(3):369-71. Wei Sheng Yan Jiu. 2004. PMID: 15211818 Review. Chinese. - The biochemical role of the heat shock protein 90 chaperone complex in establishing human telomerase activity.
Keppler BR, Grady AT, Jarstfer MB. Keppler BR, et al. J Biol Chem. 2006 Jul 21;281(29):19840-8. doi: 10.1074/jbc.M511067200. Epub 2006 May 19. J Biol Chem. 2006. PMID: 16714764
Cited by
- Chromatin structure in telomere dynamics.
Galati A, Micheli E, Cacchione S. Galati A, et al. Front Oncol. 2013 Mar 7;3:46. doi: 10.3389/fonc.2013.00046. eCollection 2013. Front Oncol. 2013. PMID: 23471416 Free PMC article. - MKRN expression pattern during embryonic and post-embryonic organogenesis in rice (Oryza sativa L. var. Nipponbare).
Wadekar HB, Sahi VP, Morita EH, Abe S. Wadekar HB, et al. Planta. 2013 Apr;237(4):1083-95. doi: 10.1007/s00425-012-1828-2. Epub 2012 Dec 23. Planta. 2013. PMID: 23262670 - A new pathway in the control of the initiation of puberty: the MKRN3 gene.
Abreu AP, Macedo DB, Brito VN, Kaiser UB, Latronico AC. Abreu AP, et al. J Mol Endocrinol. 2015 Jun;54(3):R131-9. doi: 10.1530/JME-14-0315. J Mol Endocrinol. 2015. PMID: 25957321 Free PMC article. Review. - Binding of the sphingolipid S1P to hTERT stabilizes telomerase at the nuclear periphery by allosterically mimicking protein phosphorylation.
Panneer Selvam S, De Palma RM, Oaks JJ, Oleinik N, Peterson YK, Stahelin RV, Skordalakes E, Ponnusamy S, Garrett-Mayer E, Smith CD, Ogretmen B. Panneer Selvam S, et al. Sci Signal. 2015 Jun 16;8(381):ra58. doi: 10.1126/scisignal.aaa4998. Sci Signal. 2015. PMID: 26082434 Free PMC article. - MRKNs: Gene, Functions, and Role in Disease and Infection.
Wang T, Liu W, Wang C, Ma X, Akhtar MF, Li Y, Li L. Wang T, et al. Front Oncol. 2022 Apr 8;12:862206. doi: 10.3389/fonc.2022.862206. eCollection 2022. Front Oncol. 2022. PMID: 35463379 Free PMC article. Review.
References
- Blackburn E.H. 1992. Telomerases. Annu. Rev. Biochem. 61: 113–129. - PubMed
- Blasco M.A., Lee, H.W., Hande, M.P., Samper, E., Lansdorp, P.M., DePinho, R.A., and Greider, C.W. 1997. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91: 25–34. - PubMed
- Connell P., Ballinger, C.A., Jiang, J., Wu, Y., Thompson, L.J., Hohfeld, J., and Patterson, C. 2001. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat. Cell Biol. 3: 93–96. - PubMed
- Forsythe H.L., Jarvis, J.L., Turner, J.W., Elmore, L.W., and Holt, S.E. 2001. Stable association of hsp90 and p23, but Not hsp70, with active human telomerase. J. Biol. Chem. 276: 15571–15574. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases