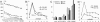Structural and numerical chromosome changes in colon cancer develop through telomere-mediated anaphase bridges, not through mitotic multipolarity - PubMed (original) (raw)
Structural and numerical chromosome changes in colon cancer develop through telomere-mediated anaphase bridges, not through mitotic multipolarity
Ylva Stewénius et al. Proc Natl Acad Sci U S A. 2005.
Abstract
Telomere dysfunction has been associated with chromosomal instability in colorectal carcinoma, but the consequences of telomere-dependent instability for chromosome integrity and clonal evolution have been little explored. We show here that abnormally short telomeres lead to a wide spectrum of mitotic disturbances in colorectal cancer cell lines, including anaphase bridging, whole-chromosome lagging, and mitotic multipolarity. These abnormalities were found in both the presence and absence of microsatellite instability. The mean telomere length varied extensively between cells from the same tumor, allowing the establishment of tumor cell subpopulations with highly different frequencies of mitotic disturbances. Anaphase bridging typically resulted in either inter-centromeric chromatin fragmentation or centromere detachment, leading to pericentromeric chromosome rearrangements and loss of whole chromosomes, respectively. There was a strong correlation between anaphase bridges and multipolar mitoses, and the induction of dicentric chromosomes by gamma irradiation and telomerase inhibition led to an elevated frequency of multipolar mitotic spindles, suggesting that multipolarity could result from polyploidization triggered by anaphase bridging. Chromatid segregation in multipolar mitoses was close to random, resulting in frequent nullisomies and nonviable daughter cells. In contrast, there was a high clonogenic survival among cells having gone through anaphase bridging in bipolar mitoses. Bridging of telomere-deficient chromosomes could thus be a major mutational mechanism in colorectal cancer, whereas mitotic multipolarity appears to be a secondary phenomenon that rarely, if ever, contributes to clonal evolution.
Figures
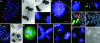
Fig. 1.
Cytogenetic analyses and single-cell cloning. (A) TTAGGG-negative ends (red arrows) and a dicentric chromosome (green arrow) in SW480. (B and C) Anaphase bridges in DLD1 (B) and SW480 (C). (D) Loss of chromosomes X (arrow; centromere in green) and 18 (centromere in violet) through bridge formation in SW480. (E and F) Fragmented DNA (green) flanked by alphoid repeats (arrows; red) in SW480. (G and H) A dicentric chromosome 7 derivative (red chromosome paint in G; red arrow in H) and two normal chromosomes 12 (green chromosome paint in G) in HT29. (I) A ring chromosome positive with the 7q31 probe (green) but not with the chromosome 7 alphoid probe (red) in the HCT116 subclone M7. (J and K) A tetrapolar anaphase cell in hematoxylin-eosin staining (J) and a tripolar metaphase cell with three centrosomes (K)(γ-tubulin in orange) in SW480. (L_–_N) FISH analyses with centromeric probes in SW480 showing a tetrapolar metaphase cell with four copies of the X chromosome (green) and six copies of chromosome 18 (violet) (L), a tripolar anaphase cell with 2 + 2 + 0 segregation of chromosome X and 3 + 2 + 1 segregation of chromosome 18 (M), and a tripolar anaphase cell with 3 + 1 + 0 segregation of chromosome 18 (N). (O) Degenerating daughter cells of an isolated tetrapolar mitotic cell from the same cell line. (Magnifications: ×1,000, A, E, and I; ×100, B_–_D, F, and J_–_N; ×2,000, G and H; and ×40, O.)
Fig. 2.
Telomere lengths, postirradiation development, and aneusomy. (A) Mean TFI measurements in normal lymphocytes and cells from three colorectal carcinoma cell lines; x axis represents rank order of cells. (B) Frequencies of cells with anaphase bridges before (passage 0) and after (passages 1–6) gamma irradiation of HCT116 cells with WT TP53 status and SW480 cells with homozygous TP53 mutation. (C) Interphase FISH assessment of the frequency of aneusomic cells using centromeric (cen) probes. (D) Frequencies of MMs before (passage 0) and after irradiation (passages 1–6) of HCT116 and SW480 cells.
Similar articles
- Telomere-mediated mitotic disturbances in immortalized ovarian epithelial cells reproduce chromosomal losses and breakpoints from ovarian carcinoma.
Gisselsson D, Lv M, Tsao SW, Man C, Jin C, Höglund M, Kwong YL, Jin Y. Gisselsson D, et al. Genes Chromosomes Cancer. 2005 Jan;42(1):22-33. doi: 10.1002/gcc.20094. Genes Chromosomes Cancer. 2005. PMID: 15390185 - Mitotic instability in cancer: is there method in the madness?
Gisselsson D. Gisselsson D. Cell Cycle. 2005 Aug;4(8):1007-10. doi: 10.4161/cc.4.8.1884. Epub 2005 Aug 1. Cell Cycle. 2005. PMID: 16082199 Review. - Different outcomes of telomere-dependent anaphase bridges.
Tusell L, Pampalona J, Soler D, Frías C, Genescà A. Tusell L, et al. Biochem Soc Trans. 2010 Dec;38(6):1698-703. doi: 10.1042/BST0381698. Biochem Soc Trans. 2010. PMID: 21118150 - Whole chromosome loss is promoted by telomere dysfunction in primary cells.
Pampalona J, Soler D, Genescà A, Tusell L. Pampalona J, et al. Genes Chromosomes Cancer. 2010 Apr;49(4):368-78. doi: 10.1002/gcc.20749. Genes Chromosomes Cancer. 2010. PMID: 20088004 - Connecting mitotic instability and chromosome aberrations in cancer--can telomeres bridge the gap?
Gisselsson D, Höglund M. Gisselsson D, et al. Semin Cancer Biol. 2005 Feb;15(1):13-23. doi: 10.1016/j.semcancer.2004.09.002. Semin Cancer Biol. 2005. PMID: 15613284 Review.
Cited by
- From aneuploidy to cancer: the evolution of a new species?
Knauss S, Klein A. Knauss S, et al. J Biosci. 2012 Jun;37(2):211-20. doi: 10.1007/s12038-012-9199-1. J Biosci. 2012. PMID: 22581326 No abstract available. - When Three Isn't a Crowd: A Digyny Concept for Treatment-Resistant, Near-Triploid Human Cancers.
Salmina K, Gerashchenko BI, Hausmann M, Vainshelbaum NM, Zayakin P, Erenpreiss J, Freivalds T, Cragg MS, Erenpreisa J. Salmina K, et al. Genes (Basel). 2019 Jul 19;10(7):551. doi: 10.3390/genes10070551. Genes (Basel). 2019. PMID: 31331093 Free PMC article. Review. - Mitotic Origins of Chromosomal Instability in Colorectal Cancer.
Dalton WB, Yang VW. Dalton WB, et al. Curr Colorectal Cancer Rep. 2007 Apr;3(2):59-64. doi: 10.1007/s11888-007-0001-y. Curr Colorectal Cancer Rep. 2007. PMID: 18843382 Free PMC article. - Somatic Sex: On the Origin of Neoplasms With Chromosome Counts in Uneven Ploidy Ranges.
Haas OA. Haas OA. Front Cell Dev Biol. 2021 Aug 4;9:631946. doi: 10.3389/fcell.2021.631946. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34422788 Free PMC article. - Cytokinesis breaks dicentric chromosomes preferentially at pericentromeric regions and telomere fusions.
Lopez V, Barinova N, Onishi M, Pobiega S, Pringle JR, Dubrana K, Marcand S. Lopez V, et al. Genes Dev. 2015 Feb 1;29(3):322-36. doi: 10.1101/gad.254664.114. Genes Dev. 2015. PMID: 25644606 Free PMC article.
References
- Cahill, D. P., Lengauer, C., Yu, J., Riggins, G. J., Willson, J. K., Markowitz, S. D., Kinzler, K. W. & Vogelstein, B. (1998) Nature 392, 300–303. - PubMed
- Rajagopalan, H., Jallepalli, P. V., Rago, C., Velculescu, V. E., Kinzler, K. W., Vogelstein, B. & Lengauer, C. (2004) Nature 428, 77–81. - PubMed
- Fodde, R., Kuipers, J., Rosenberg, C., Smits, R., Kielman, M., Gaspar, C., van Es, J. H., Breukel, C., Wiegant, J., Giles, R. H. & Clevers, H. (2001) Nat. Cell Biol. 3, 433–438. - PubMed
- Ricciardiello, L., Baglioni, M., Giovannini, C., Pariali, M., Cenacchi, G., Ripalti, A., Landini, M. P., Sawa, H., Nagashima, K., Frisque, R. J., et al. (2003) Cancer Res. 63, 7256–7262. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources