Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins - PubMed (original) (raw)
Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins
Tomoko Moriyama et al. Mol Pain. 2005.
Abstract
Prostaglandin E2 (PGE2) and prostaglandin I2 (PGI2) are major inflammatory mediators that play important roles in pain sensation and hyperalgesia. The role of their receptors (EP and IP, respectively) in inflammation has been well documented, although the EP receptor subtypes involved in this process and the underlying cellular mechanisms remain to be elucidated. The capsaicin receptor TRPV1 is a nonselective cation channel expressed in sensory neurons and activated by various noxious stimuli. TRPV1 has been reported to be critical for inflammatory pain mediated through PKA- and PKC-dependent pathways. PGE2 or PGI2increased or sensitized TRPV1 responses through EP1 or IP receptors, respectively predominantly in a PKC-dependent manner in both HEK293 cells expressing TRPV1 and mouse DRG neurons. In the presence of PGE2 or PGI2, the temperature threshold for TRPV1 activation was reduced below 35 degrees C, so that temperatures near body temperature are sufficient to activate TRPV1. A PKA-dependent pathway was also involved in the potentiation of TRPV1 through EP4 and IP receptors upon exposure to PGE2 and PGI2, respectively. Both PGE2-induced thermal hyperalgesia and inflammatory nociceptive responses were diminished in TRPV1-deficient mice and EP1-deficient mice. IP receptor involvement was also demonstrated using TRPV1-deficient mice and IP-deficient mice. Thus, the potentiation or sensitization of TRPV1 activity through EP1 or IP activation might be one important mechanism underlying the peripheral nociceptive actions of PGE2 or PGI2.
Figures
Figure 1
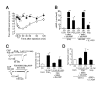
Physiological interaction of PGE2 with TRPV1 in mice. (A) PGE2-induced thermal hyperalgesia in TRPV1+/+ mice (○, n = 6) or TRPV1-/- mice (▲, n = 6). Reduction of paw withdrawal latency (thermal hyperalgesia) by intraplantar PGE2 (500 pmol/ 20 μL) injection was significantly diminished in TRPV1-/- mice. * p < 0.05, ** p < 0.01 vs. TRPV1+/+ mice. (B) Intracellular cAMP levels in mouse DRG neurons or HEK293 cells treated with a mixture of forskolin (FSK, 10 μM), IBMX (1 mM) and dibutyryl cAMP (dbcAMP, 3 mM), or PGE2 (1 μM) or isoproterenol (Isop., 10 μM). *, # p < 0.05 vs. Cont., **, ## p < 0.01 vs. Cont. (C) Representative traces of potentiation of capsaicin (100 nM)-activated current by extracellular PGE2 (1 μM, 1.5 min) or a mixture of FSK(10 μM), IBMX (1 mM) and dbcAMP (3 mM) (6.5 min) in mouse DRG neurons. Currents were normalized to values induced by first capsaicin application in the absence of additives (bar graph). Capsaicin was reapplied 1.5 or 6.5 min after exposure to bath solution with additives. Numbers in parenthesis indicate cells tested. * p < 0.05 vs. Cont. Holding potential (Vh): -60 mV. (D) Long (6.5 min) but not short (1.5 min) activation of PKA pathway has effect on TRPV1 responses in HEK293 cells. FSK (10 μM), IBMX (1 mM) and dbcAMP (3 mM) were applied to cells expressing rat TRPV1. Isop. (10 μM) was applied to cells expressing both rat TRPV1 and β1-adrenergic receptors (β1-ADR). Numbers in parenthesis indicate cells tested. Vh: -60 mV. * p < 0.05 vs Cont.
Figure 2
PGE2 increases TRPV1 activity through EP1 receptors in a PKC-dependent manner in HEK293 cells. (A) and (B) Treatment with PGE2 (1.5 min) potentiates capsaicin-evoked responses in cells expressing rat TRPV1 with mouse EP1 receptors, but not with other mouse EP receptors. Cells were pretreated with PGE2 (1 μM) for 1.5 or 6.5 min before second capsaicin (20 nM) application. Vh: -60 mV. Currents were normalized as described in Figure 1. * p < 0.05 vs. control (Cont.). Numbers in parenthesis indicate cells tested. (C) Capsaicin dose-response curves for TRPV1 activation in the absence (•) and presence (○) of extracellular 1 μM PGE2. Currents were normalized to the current maximally activated by 1 μM capsaicin in the absence of PGE2. Figure shows averaged data fitted with the Hill equation. EC50 = 81.0 nM and Hill coefficient = 1.33 in the absence of PGE2. EC50 = 27.6 nM and Hill coefficient = 1.01 in the presence of PGE2. Data were obtained from 54 different cells. (D) Temperature threshold for TRPV1 activation was reduced in the presence of extracellular PGE2 (1 μM). Representative temperature-response profiles in the absence (upper) and presence (lower) of PGE2 (left). Temperature threshold for TRPV1 activation in the presence of PGE2 (30.6 ± 1.1°C) was significantly lower than that in the absence of PGE2 (40.7 ± 0.3°C) (right). * p < 0.05 vs. PGE2 (-). Numbers in parenthesis indicate cells tested. (E) Proton-evoked TRPV1 responses were significantly potentiated by PGE2 (1 μM). * p < 0.01 vs. PGE2 (-). (F) PKC-dependent pathway is involved in the PGE2 (1 μM, 1.5 min)-induced potentiation of capsaicin-activated currents. In some experiments, calphostin C (Calp. C) (1 μM) or PKCε translocation inhibitor (PKCε-I) (200 μM) was included in the pipette solution. Currents were normalized as described in Figure 1. Numbers in parenthesis indicate cells tested. * p < 0.05 vs. Cont. Vh: -60 mV.
Figure 3
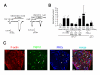
EP1 receptor involvement in PGE2 (1.5 min)-induced potentiation of capsaicin-activated currents in mouse DRG neurons. (A) Representative traces of potentiation of capsaicin-activated currents by a specific EP1 agonist, ONO-DI-004 (10 μM, 1.5 min), and reverse of the PGE2 (1.5 min)-induced potentiation by a specific EP1 antagonist, ONO-8713 (1 μM). Vh: -60 mV. (B) Effects of PGE2 (1 μM), ONO-DI-004 (EP1 Agon., 10 μM), PGE2 plus ONO-8713 (EP1 Antg., 1 μM), PGE2 plus U73122 (3 μM), PGE2 plus U73343 (3 μM), phorbol 12-myristate 13-acetate (PMA, 100 nM) or PGE2 plus PKCε-I (200 μM) on capsaicin-activated currents in DRG neurons from wild type (EP1+/+) mice, and effects of PGE2 on capsaicin-activated currents in DRG neurons from EP1-/- mice. Currents are normalized as described in Fig. 1. * p < 0.05 vs. Cont., + p < 0.05 vs. U73343. Numbers in parenthesis indicate cells tested. (C) Co-expression of TRPV1 (green) and PKCε (blue) in mouse DRG. Arrowheads indicate neurons positive for TRPV1 but not for PKCε. Arrows indicate neurons positive for both TRPV1 and PKCε (light blue). Bar, 100 μm.
Figure 4
Interaction between TRPV1 and EP1 receptors in a behavioral level. (A) PGE2-induced thermal hyperalgesia in wild type mice with (▴, n = 6) or without (○, n = 6) pretreatment (ONO-8713, 500 pmol/ 20 μL), or in EP1-/- mice (•, n = 6). * p < 0.05 vs. wild type mice. (B) 10% Mustard oil-induced thermal hyperalgesia in wild type mice (○, n = 12), TRPV1-/- mice (▲, n = 6) or EP1-/- mice (•, n = 6). * p < 0.05, ** p < 0.01 vs. wild type mice.
Figure 5
PGI2 causes potentiation or sensitization of TRPV1 through mainly through PKC activation. (A) Representative traces of potentiation of capsaicin-activated currents by PGI2 (1000 nM, 1.5 min), a specific IP agonist, ONO-54918-07 (1.5 min) or PGI2 (100 nM, 6.5 min), but not by PGI2 (100 nM, 1.5 min) in mouse DRG neurons. Vh: -60 mV. (B) Effects of treatments (1.5 or 6.5 min) with PGI2 (100 or 1000 nM), ONO-54918-07 (IP Agon., 100 nM), PGI2 (1000 nM) plus ONO-8713 (EP1 Antg., 1 μM), PGI2 (1000 nM) plus U73122 (3 μM), PGI2 (1000 nM) plus U73343 (3 μM) or PGI2 (1000 nM) plus PKCε-I (200 μM) on capsaicin-activated currents in DRG neurons from wild type (IP+/+) mice, and effects of PGI2 on capsaicin-activated currents in DRG neurons from IP-deficient (IP-/-) mice. Currents are normalized as described in Figure 1. * p < 0.05 vs. Cont. ++ p < 0.01 vs. U73343, # p < 0.05, ## p < 0.01 vs. PGI2 (1000 nM, 1.5 min) in DRG neurons from IP+/+ mice. Numbers in parenthesis indicate cells tested. (C) A representative trace of potentiation of capsaicin-activated currents by PGI2 (1000 nM, 1.5 min) in HEK293 cells expressing both TRPV1 and IP. Vh: -60 mV. (D) Effects of treatments (1.5 or 6.5 min) with PGI2 (100 or 1000 nM) or PGI2 (1000 nM) plus calphostin C (Calp. C, 1 μM) on capsaicin-activated currents in HEK293 cells expressing rat wild type TRPV1 or S502A/S800A mutant with IP. Currents are normalized as described in Figure 1. * p < 0.05 vs. Cont. (E) Temperature threshold for TRPV1 activation in the presence of PGI2 (32.2 ± 1.2°C) was significantly lower than that in the absence of PGI2 (38.2 ± 0.5°C) in HEK293 cells expressing rat TRPV1 and IP. * p < 0.01 vs. PGI2 (-).
Figure 6
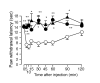
Interaction between TRPV1 and IP receptors at a behavioral level. PGI2-induced thermal hyperalgesia in wild type mice (○, n = 6), TRPV1-/- mice (▲, n = 6) or IP-/- mice (•, n = 6). Thermal hyperalgesia by intraplantar PGI2 (500 pmol/ 20 μL) injection was significantly diminished in TRPV1-/- mice and IP-/- mice. * p < 0.05, ** p < 0.01 vs. wild type mice.
Similar articles
- Stimulating TRPV1 externalization and synthesis in dorsal root ganglion neurons contributes to PGE2 potentiation of TRPV1 activity and nociceptor sensitization.
Ma W, St-Jacques B, Rudakou U, Kim YN. Ma W, et al. Eur J Pain. 2017 Apr;21(4):575-593. doi: 10.1002/ejp.959. Epub 2016 Oct 14. Eur J Pain. 2017. PMID: 27739618 - Enhancement of acid-sensing ion channel activity by prostaglandin E2 in rat dorsal root ganglion neurons.
Zhou YM, Wu L, Wei S, Jin Y, Liu TT, Qiu CY, Hu WP. Zhou YM, et al. Brain Res. 2019 Dec 1;1724:146442. doi: 10.1016/j.brainres.2019.146442. Epub 2019 Sep 9. Brain Res. 2019. PMID: 31513790 - [Physiological functions of prostanoid receptors and their subtypes].
Sugimoto Y. Sugimoto Y. Nihon Yakurigaku Zasshi. 2000 Mar;115(3):131-41. doi: 10.1254/fpj.115.131. Nihon Yakurigaku Zasshi. 2000. PMID: 10876798 Review. Japanese. - Prostaglandin EP receptors and their roles in mucosal protection and ulcer healing in the gastrointestinal tract.
Takeuchi K. Takeuchi K. Adv Clin Chem. 2010;51:121-44. doi: 10.1016/s0065-2423(10)51005-9. Adv Clin Chem. 2010. PMID: 20857620 Review.
Cited by
- Dry eye modifies the thermal and menthol responses in rat corneal primary afferent cool cells.
Kurose M, Meng ID. Kurose M, et al. J Neurophysiol. 2013 Jul;110(2):495-504. doi: 10.1152/jn.00222.2013. Epub 2013 May 1. J Neurophysiol. 2013. PMID: 23636717 Free PMC article. - Novel Analgesics with Peripheral Targets.
Ciotu CI, Fischer MJM. Ciotu CI, et al. Neurotherapeutics. 2020 Jul;17(3):784-825. doi: 10.1007/s13311-020-00937-z. Epub 2020 Oct 15. Neurotherapeutics. 2020. PMID: 33063247 Free PMC article. Review. - Nociceptors in cardiovascular functions: complex interplay as a result of cyclooxygenase inhibition.
Premkumar LS, Raisinghani M. Premkumar LS, et al. Mol Pain. 2006 Aug 17;2:26. doi: 10.1186/1744-8069-2-26. Mol Pain. 2006. PMID: 16916451 Free PMC article. Review. - Anoctamin 1 contributes to inflammatory and nerve-injury induced hypersensitivity.
Lee B, Cho H, Jung J, Yang YD, Yang DJ, Oh U. Lee B, et al. Mol Pain. 2014 Jan 23;10:5. doi: 10.1186/1744-8069-10-5. Mol Pain. 2014. PMID: 24450308 Free PMC article. - Immediate and delayed potentiating effects of tumor necrosis factor-α on TRPV1 sensitivity of rat vagal pulmonary sensory neurons.
Hsu CC, Lin YS, Lin RL, Lee LY. Hsu CC, et al. Am J Physiol Lung Cell Mol Physiol. 2017 Aug 1;313(2):L293-L304. doi: 10.1152/ajplung.00235.2016. Epub 2017 May 18. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28522561 Free PMC article.
References
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases