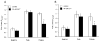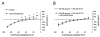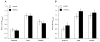Membrane hyperpolarization is not required for sustained muscarinic agonist-induced increases in intracellular Ca2+ in arteriolar endothelial cells - PubMed (original) (raw)
Membrane hyperpolarization is not required for sustained muscarinic agonist-induced increases in intracellular Ca2+ in arteriolar endothelial cells
Kenneth D Cohen et al. Microcirculation. 2005 Mar.
Abstract
Objective: Hyperpolarization modulates Ca2+ influx during agonist stimulation in many endothelial cells, but the effects of hyperpolarization on Ca2+ influx in freshly isolated arteriolar endothelial cells are unknown. Therefore, the purpose of the present study was to characterize agonist-induced Ca2+ transients in freshly isolated arteriolar endothelial cells and to test the hypothesis that membrane hyperpolarization augments agonist-induced Ca2+ influx into these cells.
Methods: Arterioles were removed from hamster cremaster muscles and arteriolar endothelial cells were enzymatically isolated. Endothelial cells were loaded with Fura 2-AM and the Fura 2 ratio measured photometrically as an index of intracellular Ca2+. The cells were then stimulated with the muscarinic, cholinergic agonist, methacholine, and the resulting Ca2+ transients were measured.
Results: Methacholine (1 microM) increased the endothelial cell Fura 2 ratio from a baseline of 0.81 +/- 0.02 to an initial peak of 1.17 +/- 0.05 (n = 17) followed by a sustained plateau of 1.12 +/- 0.07. The plateau phase of the Ca2+ transient was inhibited by removal of extracellular Ca2+ (n = 12, p < .05), or the nonselective cation channel blockers Gd3+ (30 microM; n = 7, p < .05) or La3+ (50 microM; n = 7, p < .05) without significant effect on the baseline or peak (p > .05). The initial peak of methacholine-induced Ca2+ transients was inhibited by the IP3-receptor antagonist xestospongin D (10 microM, n = 5, p < .05). The methacholine-induced Ca2+ transients were accompanied by endothelial cell hyperpolarization of approximately 14-18 mV, as assessed by experiments using the potentiometric dye, di-8-ANEPPS as well as by patch-clamp experiments. However, inhibition of hyperpolarization by blockade of Ca2+-activated K+ channels with charybdotoxin (100 nM) and apamin (100 nM) (n = 5), or exposure of endothelial cells to 80 or 145 mM KCl (both n = 7) had no effect on the plateau phase of methacholine-induced Ca2+ transients (p > .05).
Conclusions: Freshly isolated arteriolar endothelial cells display agonist-induced Ca2+ transients. For the muscarinic agonist, methacholine, these Ca2+ transients result from release of Ca2+ from intracellular stores through IP3 receptors, followed by sustained influx of extracellular Ca2+. While these changes in intracellular Ca2+ are associated with endothelial cell hyperpolarization, the methacholine-induced, sustained increase in intracellular Ca2+ appears to be independent from this change in membrane potential. These data suggest that arteriolar endothelial cells may possess a novel Ca2+ influx pathway, or that the relationship between intracellular Ca2+ and Ca2+ influx is more complex than that observed in other endothelial cells.
Figures
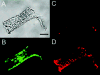
Figure 1
Arteriolar endothelial cell tubes take up and hydrolyze calcein-AM while excluding ethidium homodimer-1. (A) Transmitted light image of an endothelial cell tube from a hamster cremaster arteriole. Cells were incubated in calcein-AM (1 μM) and ethidium homodimer-1 (1 μM) for 15 min. The bar in this panel represents 50 μm. (B) Fluorescent image of the same endothelial cell tube as in (A) that has taken up and cleaved calcein-AM (excitation wavelength = 494 nm, emission wavelength = 520 nm). (C) Fluorescent image of the same endothelial cell tube as in panels (A) and (B) demonstrating exclusion of ethidium homodimer-1 (excitation wavelength = 528 nm, emission wavelength = 617 nm). (D) Fluorescent image of the same endothelial cell tube as in (A)–(C) after exposure to 0.1% Triton X-100 to permeabilize the cell membranes and demonstrate positive ethidium homodimer-1 staining (excitation wavelength = 528 nm, emission wavelength = 617 nm).
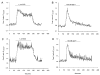
Figure 2
Agonist-induced Ca2+ transients in isolated arteriolar endothelial cell tubes are similar to responses of endothelial cells in an intact arteriole. Representative traces of the Fura 2 ratio in isolated arteriolar endothelial cells in response to 1 μM methacholine in isolated arteriolar endothelial cells (A) and in endothelial cells from an intact arteriole (B). Representative traces of the Fura 2 ratio in isolated arteriolar endothelial cells in response to 100 nM substance P in isolated arteriolar endothelial cells (C) and in endothelial cells from an intact arteriole (D). Methacholine (MCh) or substance P (sub P) application is denoted by a line in each figure.
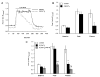
Figure 3
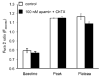
Removal of [Ca2+]o inhibits methacholine-induced sustained increases in intracellular Ca2+ in isolated arteriolar endothelial cell tubes. (A) Representative traces of the Fura 2 ratio in endothelial cell tubes in response to methacholine in the presence (solid line) and absence (dotted line) of [Ca2+]o. (B) Summary data (n = 12) of the baseline, peak and plateau Fura 2 ratio values in response to methacholine in the presence (open bars) and absence (closed bars) of extracellular Ca2+. (C) Summary data (n = 5) of the baseline, peak, and plateau Fura 2 values in response to methacholine under control conditions (open bars), in the absence of [Ca2+]o (closed bars) and in the absence of [Ca2+]o but now in the presence of 10 μM XSP D (gray bars). *Significantly different from control at that same component of the Ca2+ transient; #significantly different from 0 [Ca2+]o ( p < .05 for both).
Figure 4
The inorganic cation channel blockers, La3+ and Gd3+, block the methacholine-induced sustained increases in intracellular Ca2+ in isolated arteriolar endothelial cells. Summary data of the baseline, peak and plateau Fura 2 values in isolated arteriolar endothelial cells in response to methacholine under control conditions (open bars) and in the presence of 30 μM Gd3+ (A; closed bars; n = 7) and 50 μM La3+ (B; closed bars; n = 7).
Figure 5
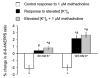
The combination of charybdotoxin and apamin did not inhibit the methacholine-induced Ca2+ transients in isolated arteriolar endothelial cells (n = 5). Summary data of the baseline, peak and plateau Fura 2 values in isolated arteriolar endothelial cells in response to methacholine under control conditions (open bars) and in the presence of 100 nM charybdotoxin and apamin (closed bars)
Figure 6
Charybdotoxin and apamin inhibit methacholine-induced K+ currents. (A) Current–voltage plots of whole endothelial cell K+ currents under control conditions (open circles) and in response to 1 μM methacholine (closed circles). (B) Current–voltage plots of whole endothelial cell K+ currents in the presence of 100 nM charybdotoxin and apamin (open circles) and in the presence of charybdotoxin, apamin and 1 μM methacholine (closed circles). *Significantly different from currents in the absence of methacholine (p < .05).
Figure 7
High [K+]o solutions did not inhibit the methacholine-induced Ca2+ transients. Summary data of the baseline, peak, and plateau Fura 2 values in isolated arteriolar endothelial cells in response to methacholine under control conditions (open bars) and in the presence of 80 mM [K+]o (A; closed bars; n = 7) and 145 mM [K+]o (B; closed bars; n = 6).
Figure 8
Methacholine hyperpolarizes freshly isolated arteriolar endothelial cells and this hyperpolarization is inhibited by high [K+]o. Summary data of the di-8-ANEPPS ratio in arteriolar endothelial cells corresponding (in time) to the plateau of the Fura 2 ratio changes in response to methacholine during exposure of arteriolar endothelial cells to 80 or 145 mM [K+]o, as indicated. *Significant change from baseline (p < .05); #significantly different from methacholine alone (p < .05).
Similar articles
- Measurement of membrane potential and intracellular Ca(2+) of arteriolar endothelium and smooth muscle in vivo.
Chen Y, Rivers RJ. Chen Y, et al. Microvasc Res. 2001 Jul;62(1):55-62. doi: 10.1006/mvre.2001.2315. Microvasc Res. 2001. PMID: 11421660 - K+-induced dilation of hamster cremasteric arterioles involves both the Na+/K+-ATPase and inward-rectifier K+ channels.
Burns WR, Cohen KD, Jackson WF. Burns WR, et al. Microcirculation. 2004 Apr-May;11(3):279-93. doi: 10.1080/10739680490425985. Microcirculation. 2004. PMID: 15280082 Free PMC article. - Hypoxia inhibits contraction but not calcium channel currents or changes in intracellular calcium in arteriolar muscle cells.
Cohen KD, Jackson WF. Cohen KD, et al. Microcirculation. 2003 Apr;10(2):133-41. doi: 10.1038/sj.mn.7800178. Microcirculation. 2003. PMID: 12700582 Free PMC article. - Ion channels and regulation of intracellular calcium in vascular endothelial cells.
Adams DJ, Barakeh J, Laskey R, Van Breemen C. Adams DJ, et al. FASEB J. 1989 Oct;3(12):2389-400. doi: 10.1096/fasebj.3.12.2477294. FASEB J. 1989. PMID: 2477294 Review. - [Hyperpolarization-relaxation coupling in vascular smooth muscle].
Yanagisawa T. Yanagisawa T. Nihon Yakurigaku Zasshi. 1995 Sep;106(3):157-69. doi: 10.1254/fpj.106.157. Nihon Yakurigaku Zasshi. 1995. PMID: 8529961 Review. Japanese.
Cited by
- Integration and Modulation of Intercellular Signaling Underlying Blood Flow Control.
Segal SS. Segal SS. J Vasc Res. 2015;52(2):136-57. doi: 10.1159/000439112. J Vasc Res. 2015. PMID: 26368324 Free PMC article. - Boosting the signal: Endothelial inward rectifier K+ channels.
Jackson WF. Jackson WF. Microcirculation. 2017 Apr;24(3):10.1111/micc.12319. doi: 10.1111/micc.12319. Microcirculation. 2017. PMID: 27652592 Free PMC article. Review. - Functional coupling of TRPV4, IK, and SK channels contributes to Ca(2+)-dependent endothelial injury in rodent lung.
Lin MT, Jian MY, Taylor MS, Cioffi DL, Yap FC, Liedtke W, Townsley MI. Lin MT, et al. Pulm Circ. 2015 Jun;5(2):279-90. doi: 10.1086/680166. Pulm Circ. 2015. PMID: 26064452 Free PMC article. - Heterogeneous function of ryanodine receptors, but not IP3 receptors, in hamster cremaster muscle feed arteries and arterioles.
Westcott EB, Jackson WF. Westcott EB, et al. Am J Physiol Heart Circ Physiol. 2011 May;300(5):H1616-30. doi: 10.1152/ajpheart.00728.2010. Epub 2011 Feb 25. Am J Physiol Heart Circ Physiol. 2011. PMID: 21357503 Free PMC article. - Phospholipase C-delta extends intercellular signalling range and responses to injury-released growth factors in non-excitable cells.
Mi LY, Ettenson DS, Edelman ER. Mi LY, et al. Cell Prolif. 2008 Aug;41(4):671-90. doi: 10.1111/j.1365-2184.2008.00544.x. Epub 2008 Jun 19. Cell Prolif. 2008. PMID: 18616695 Free PMC article.
References
- Adams DJ, Barakeh J, Laskey R, Van Breemen C. Ion channels and regulation of intracellular calcium in vascular endothelial cells. FASEB J. 1989;3:2389–2400. - PubMed
- Ay B, Prakash YS, Pabelick CM, Sieck GC. Store-operated Ca2+ entry in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2004;286:L909–L917. - PubMed
- Busse R, Pohl U, Luckhoff A. Mechanisms controlling the production of endothelial autacoids. Z Kardiol. 1989;78 (Suppl 6):64–69. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous