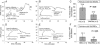Hemoglobin conformation couples erythrocyte S-nitrosothiol content to O2 gradients - PubMed (original) (raw)
. 2005 Apr 19;102(16):5709-14.
doi: 10.1073/pnas.0407490102. Epub 2005 Apr 11.
Ruth Platt, Mary Lynn Sheram, Anne Eischeid, Timothy McMahon, Thomas Maxey, Joseph Doherty, Mark Axelrod, Jaclyn Kline, Matthew Gurka, Andrew Gow, Benjamin Gaston
Affiliations
- PMID: 15824313
- PMCID: PMC556285
- DOI: 10.1073/pnas.0407490102
Hemoglobin conformation couples erythrocyte S-nitrosothiol content to O2 gradients
Allan Doctor et al. Proc Natl Acad Sci U S A. 2005.
Abstract
It is proposed that the bond between nitric oxide (NO) and the Hb thiol Cys-beta(93) (SNOHb) is favored when hemoglobin (Hb) is in the relaxed (R, oxygenated) conformation, and that deoxygenation to tense (T) state destabilizes the SNOHb bond, allowing transfer of NO from Hb to form other (vasoactive) S-nitrosothiols (SNOs). However, it has not previously been possible to measure SNOHb without extensive Hb preparation, altering its allostery and SNO distribution. Here, we have validated an assay for SNOHb that uses carbon monoxide (CO) and cuprous chloride (CuCl)-saturated Cys. This assay is specific for SNOs and sensitive to 2-5 pmol. Uniquely, it measures the total SNO content of unmodified erythrocytes (RBCs) (SNO(RBC)), preserving Hb allostery. In room air, the ratio of SNO(RBC) to Hb in intact RBCs is stable over time, but there is a logarithmic loss of SNO(RBC) with oxyHb desaturation (slope, 0.043). This decay is accelerated by extraerythrocytic thiol (slope, 0.089; P < 0.001). SNO(RBC) stability is uncoupled from O(2) tension when Hb is locked in the R state by CO pretreatment. Also, SNO(RBC) is increased approximately 20-fold in human septic shock (P = 0.002) and the O(2)-dependent vasoactivity of RBCs is affected profoundly by SNO content in a murine lung bioassay. These data demonstrate that SNO content and O(2) saturation are tightly coupled in intact RBCs and that this coupling is likely to be of pathophysiological significance.
Figures
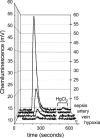
Fig. 6.
Raw chemiluminescence signals from RBCs (1 mM Hb ± HgCl2) from individual experiments shown in Figs. 3 and 4. Baselines are normalized for comparison. Overall data ranges are given in Table 2.
Fig. 4.
In patients meeting consensus criteria for SIRS and ARDS, the mean mixed venous SNORBC/Hb ratio was 1.48 × 10–3± 7.9 × 10–4, which is 21-fold higher than in normal volunteers (7.07 × 10–5± 4.3 × 10–5; P = 0.002).
Fig. 1.
Chemiluminescence signal after assay in 3C for paired injections, as indicated, of a GSNO dilution series (without CO) (series A), or the same injections of GSNO as for series A after preinjection of NO-depleted Hb into the reflux chamber (series B). The mixture of Hb and GSNO in the reflux chamber simulates the noted SNO/Hbs; the signal for GSNO is lost, presumably to capture of NO by heme Fe. (series C) The signal for GSNO as in series A returns after adding carbonyl-purged CO to the inert gas stream, resolving the signal attenuation shown in series B.
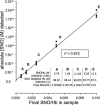
Fig. 2.
Detection (3C assay) of SNO is linear over varying SNO/Hb ratios. SNO/Hb was varied systematically by dilution of SNOHb into Hb, as noted in Inset. Absolute [SNO] detected is plotted against final SNO/Hb in the sample mixtures; detection is linear across final SNO/Hb, which varied from 0.01 to 0.00062.
Fig. 3.
SNORBC and O2 content are coupled. (A) Washed RBCs from normal humans suspended with (○) or without (•) extracellular GSH were steadily deoxygenated under argon. The SNORBC/Hb ratio is plotted against Hb SO2. (Inset) Washed RBCs without extracellular GSH were treated in the same fashion as described for A but not deoxygenated. The ratio of SNORBC to Hb was stable over time. (B) The natural logarithm of the ratio of SNORBC to Hb was modeled as a function of Hb SO2; extraerythrocytic GSH was included as a covariate, generating two lines describing the decay rate of the ratio of SNORBC to Hb with or without extraerythrocytic GSH. These rates differed (P < 0.0001).
Fig. 5.
Bioassay for O2-dependent SNORBC vasoactivity in the isolated mouse lung. Representative PAP traces during perfusion with free Hb (A), free SNOHb (B), RBCs (D), and SNORBCs (E). (A) Experimental stages are identified including (a) PA cannulation and baseline during buffer perfusion and normoxic ventilation; (b) hypoxic challenge; (c) reestablishment of baseline after normoxic ventilation; (d) new baseline after addition of free Hb or RBCs to perfusate; (e) second hypoxic challenge; and (f) reestablishment of baseline after normoxic ventilation. Note (i) an increase in baseline PAP with Hb (free Hb ≫ RBCs); (ii) no change in baseline PAP (normoxia) on SNO loading of Hb (free or RBCs); (iii) HPV amplitude during perfusion with Hb ≫ RBCs (equimolar Hb); (iv) SNO loading of either RBCs or free Hb attenuates the HPV response (C and F). SNO loading causes an O2-dependent reversal of Hb and RBC vasoactivity, constriction in normoxia is maintained (baseline pressor response is unaltered), and dilation in hypoxia emerges (HPV is blunted with SNO loading).
Similar articles
- Red Blood Cell-Mediated S-Nitrosohemoglobin-Dependent Vasodilation: Lessons Learned from a β-Globin Cys93 Knock-In Mouse.
Premont RT, Reynolds JD, Zhang R, Stamler JS. Premont RT, et al. Antioxid Redox Signal. 2021 Apr 20;34(12):936-961. doi: 10.1089/ars.2020.8153. Epub 2020 Jul 23. Antioxid Redox Signal. 2021. PMID: 32597195 Free PMC article. Review. - An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate.
Angelo M, Singel DJ, Stamler JS. Angelo M, et al. Proc Natl Acad Sci U S A. 2006 May 30;103(22):8366-71. doi: 10.1073/pnas.0600942103. Epub 2006 May 22. Proc Natl Acad Sci U S A. 2006. PMID: 16717191 Free PMC article. - Protein disulfide isomerase may facilitate the efflux of nitrite derived S-nitrosothiols from red blood cells.
Kallakunta VM, Slama-Schwok A, Mutus B. Kallakunta VM, et al. Redox Biol. 2013 Jul 16;1(1):373-80. doi: 10.1016/j.redox.2013.07.002. eCollection 2013. Redox Biol. 2013. PMID: 24024174 Free PMC article. - Evolution of adverse changes in stored RBCs.
Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM, McMahon TJ. Bennett-Guerrero E, et al. Proc Natl Acad Sci U S A. 2007 Oct 23;104(43):17063-8. doi: 10.1073/pnas.0708160104. Epub 2007 Oct 11. Proc Natl Acad Sci U S A. 2007. PMID: 17940021 Free PMC article. - Role of Nitric Oxide Carried by Hemoglobin in Cardiovascular Physiology: Developments on a Three-Gas Respiratory Cycle.
Premont RT, Reynolds JD, Zhang R, Stamler JS. Premont RT, et al. Circ Res. 2020 Jan 3;126(1):129-158. doi: 10.1161/CIRCRESAHA.119.315626. Epub 2019 Oct 8. Circ Res. 2020. PMID: 31590598 Free PMC article. Review.
Cited by
- Silent hypoxia: higher NO in red blood cells of COVID-19 patients.
Mortaz E, Malkmohammad M, Jamaati H, Naghan PA, Hashemian SM, Tabarsi P, Varahram M, Zaheri H, Chousein EGU, Folkerts G, Adcock IM. Mortaz E, et al. BMC Pulm Med. 2020 Oct 16;20(1):269. doi: 10.1186/s12890-020-01310-8. BMC Pulm Med. 2020. PMID: 33066765 Free PMC article. - Chemical and functional aspects of posttranslational modification of proteins.
Knorre DG, Kudryashova NV, Godovikova TS. Knorre DG, et al. Acta Naturae. 2009 Oct;1(3):29-51. Acta Naturae. 2009. PMID: 22649613 Free PMC article. - Lack of allosterically controlled intramolecular transfer of nitric oxide from the heme to cysteine in the beta subunit of hemoglobin.
Huang KT, Azarov I, Basu S, Huang J, Kim-Shapiro DB. Huang KT, et al. Blood. 2006 Apr 1;107(7):2602-4. doi: 10.1182/blood-2005-10-4104. Epub 2005 Dec 8. Blood. 2006. PMID: 16339397 Free PMC article. - SNO-hemoglobin is not essential for red blood cell-dependent hypoxic vasodilation.
Isbell TS, Sun CW, Wu LC, Teng X, Vitturi DA, Branch BG, Kevil CG, Peng N, Wyss JM, Ambalavanan N, Schwiebert L, Ren J, Pawlik KM, Renfrow MB, Patel RP, Townes TM. Isbell TS, et al. Nat Med. 2008 Jul;14(7):773-7. doi: 10.1038/nm1771. Epub 2008 May 30. Nat Med. 2008. PMID: 18516054 Free PMC article. - Specific Etiologies Associated With the Multiple Organ Dysfunction Syndrome in Children: Part 2.
Upperman JS, Bucuvalas JC, Williams FN, Cairns BA, Cox CS Jr, Doctor A, Tamburro RF. Upperman JS, et al. Pediatr Crit Care Med. 2017 Mar;18(3_suppl Suppl 1):S58-S66. doi: 10.1097/PCC.0000000000001051. Pediatr Crit Care Med. 2017. PMID: 28248835 Free PMC article. Review.
References
- Gow, A. & Stamler, J. (1998) Nature 391, 169–173. - PubMed
- Jia, L., Bonaventura, C., Bonaventura, J. & Stamler, J. (1996) Nature 380, 221–226. - PubMed
- Stamler, J. S. (1994) Cell 78, 931–936. - PubMed
- Stamler, J., Singel, D. & Loscalzo, J. (1992) Science 258, 1898–1902. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5K12 HD 01421-01/HD/NICHD NIH HHS/United States
- 2R01 HL 59337/HL/NHLBI NIH HHS/United States
- 1K08 GM 069977-01/GM/NIGMS NIH HHS/United States
- K12 HD001421/HD/NICHD NIH HHS/United States
- K08 GM069977/GM/NIGMS NIH HHS/United States
- R01 HL059337/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources