MicroRNA-directed regulation of Arabidopsis AUXIN RESPONSE FACTOR17 is essential for proper development and modulates expression of early auxin response genes - PubMed (original) (raw)
MicroRNA-directed regulation of Arabidopsis AUXIN RESPONSE FACTOR17 is essential for proper development and modulates expression of early auxin response genes
Allison C Mallory et al. Plant Cell. 2005 May.
Abstract
The phytohormone auxin plays critical roles during plant growth, many of which are mediated by the auxin response transcription factor (ARF) family. MicroRNAs (miRNAs), endogenous 21-nucleotide riboregulators, target several mRNAs implicated in auxin responses. miR160 targets ARF10, ARF16, and ARF17, three of the 23 Arabidopsis thaliana ARF genes. Here, we describe roles of miR160-directed ARF17 posttranscriptional regulation. Plants expressing a miRNA-resistant version of ARF17 have increased ARF17 mRNA levels and altered accumulation of auxin-inducible GH3-like mRNAs, YDK1/GH3.2, GH3.3, GH3.5, and DFL1/GH3.6, which encode auxin-conjugating proteins. These expression changes correlate with dramatic developmental defects, including embryo and emerging leaf symmetry anomalies, leaf shape defects, premature inflorescence development, altered phyllotaxy along the stem, reduced petal size, abnormal stamens, sterility, and root growth defects. These defects demonstrate the importance of miR160-directed ARF17 regulation and implicate ARF17 as a regulator of GH3-like early auxin response genes. Many of these defects resemble phenotypes previously observed in plants expressing viral suppressors of RNA silencing and plants with mutations in genes important for miRNA biogenesis or function, providing a molecular rationale for phenotypes previously associated with more general disruptions of miRNA function.
Figures
Figure 1.
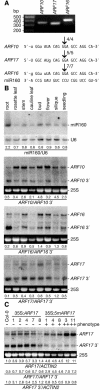
miR160 Regulates ARF10, ARF16, and ARF17 in Vivo. (A) miR160 cleavage sites in ARF10, ARF16, and ARF17 mRNAs determined by RNA ligase-mediated 5′ RACE. The agarose gel shows the nested PCR products representing the 3′ cleavage fragments that were cloned and sequenced for each gene. The frequency of 5′ RACE clones corresponding to each cleavage site (arrows) is shown as a fraction, with the number of clones matching the target message in the numerator. As expected for miRNA-directed cleavage (Llave et al., 2002), the clones ended at the nucleotide that pairs with the 10th nucleotide of miR160. Mismatched nucleotides are in lowercase letters. (B) miR160 and ARF10, ARF16, and ARF17 mRNAs accumulate differentially in Arabidopsis (Col-0) tissues. Shown is an RNA gel blot analysis of 30 μg total RNA prepared from root, rosette leaf, stem, cauline leaf, buds and inflorescence meristems (bud), flower, seedpods and embryos (silique), and 12-d-old seedling tissues with a DNA probe complementary to miR160. The blot was stripped and reprobed with an oligonucleotide complementary to U6 as a loading control. Also shown is RNA gel blot analysis of 7 μg of the same RNA with RNA probes complementary to 3′ ARF10, 3′ ARF16, or 3′ ARF17. The positions of full-length mRNAs and 3′ cleavage products are noted at the right. Ethidium bromide staining of 25S rRNA is shown as a loading control. Ratios of miR160 to U6 RNA or ARF full-length mRNA to 3′ cleavage product are indicated below each lane. (C) RNA gel blot analysis of 10 μg total RNA from rosette leaves of wild-type Col-0, 35S:ARF17, and 35S:5mARF17 T1 plants with a 3′ ARF17 probe. The absence (−) or presence of a moderate (+) or severe (++) rosette leaf phenotype and individual transformant numbers are indicated above the lanes. The positions of full-length ARF17 mRNA and ARF17 3′ cleavage product are noted at the right. The 25S rRNA is shown as a loading control. The blot was rehybridized with an ACTIN2 probe, and normalized values of ARF17 full-length mRNA and ARF17 3′ cleavage product to ACTIN2 (At3g18780) RNA (with Col-0 levels set at 1.0) and ratios of ARF17 full-length mRNA to 3′ cleavage product are indicated.
Figure 2.
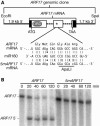
5mARF17 RNA Is Resistant to miR160-Directed Cleavage. (A) Illustration of the ARF17 genomic clone used to transform Arabidopsis. Locations of the intergenic sequences (solid lines), introns (dashed lines), 5′ and 3′ UTRs (black boxes), DBD, remainder of the ARF17 open reading frame (open boxes), start (ATG) and stop (TAA) codons, miR160 complementary site (asterisk), and the _Eco_RI and _Spe_I sites used for cloning are shown. mRNA segments from ARF17 and 5mARF17 are shown paired with miR160, with the _Apa_LI restriction site unique to 5mARF17 and the protein sequence encoded by the miR160 complementary sites of both mRNAs indicated. (B) ARF17 RNA cleavage directed by miR160 in wheat germ extracts. The 5′-radiolabeled transcripts prepared from wild-type ARF17 and 5mARF17 constructs were introduced into wheat germ extracts, and the time course of cleavage was examined on a denaturing polyacrylamide gel. The positions of ARF17 RNA and 5′ ARF17 cleavage product are noted at the left.
Figure 3.
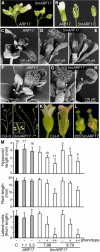
Embryonic and Vegetative Developmental Defects of 5mARF17 Plants. (A) Eight-day-old control ARF17 and 5mARF17 T2 seedlings displaying a range of embryonic phenotypes, including lobed cotyledons (bottom left of 5mARF17 panel) and extra cotyledons indicative of trilateral and quadrilateral embryonic symmetry (right 5mARF17 panels). (B) Scanning electron micrographs of a 6-d-old control ARF17 (left) plant with the normal two cotyledons (asterisks) and two emerging true leaves (arrowheads) and 10-d-old 5mARF17 seedlings (middle and right). The middle plant displays a lobed cotyledon and a downwardly curled cotyledon, and the right plant has three cotyledons (asterisks) and three emerging true leaves (trilateral symmetry). (C) Scanning electron micrographs of a 10-d-old 5mARF17 seedling (left), 6-d-old 5mARF17 seedling (middle), and magnified view of the 6-d-old 5mARF17 shoot apex (right). Indicative of quadrilaterally symmetric embryos, the 5mARF17 seedlings had four cotyledons (asterisks) and four emerging rosette leaves (arrowheads) rather than two of each. (D) Scanning electron micrographs of abaxial surfaces of 6-d-old control ARF17 and 5mARF17 cotyledons showing altered epidermal cell shape and organization in the 5mARF17 cotyledon. (E) Twenty-two-day-old control ARF17 (left) and 5mARF17 (top right) T2 plants and a 30-d-old 5mARF17 T2 plant with severe leaf serration (bottom right). (F) Rosette leaves from 30-d-old control ARF17 (left) and 5mARF17 (right) T2 plants. (G) Thirty-two-day-old control ARF17 (left) and 5mARF17 (right) T1 plants. The 5mARF17 plant has bolted. (H) Magnified view of 5mARF17 plant in (G). (I) Seventy-two-day-old control ARF17 and three 5mARF17 T2 plants grown in short days (8 h light, 16 h dark). The 5mARF17 developmental defects are increasing in severity from left to right; only the plant with the most severe phenotype has bolted. (J) Col-0 and 35S:5mARF17 rosette leaves with defects increasing in severity from left to right.
Figure 4.
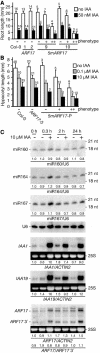
Floral Organ and Seedling Defects of 5mARF17 Plants. (A) Side view of the inflorescence bud cluster of ARF17 and 5mARF17 T1 plants. (B) Mature ARF17 and 5mARF17 T2 flowers with 5mARF17 petal and stamen defects increasing in severity from left to right. (C) to (E) Scanning electron micrographs of an ARF17 T2 mature flower (C) and 5mARF17 T2 mature flowers with moderate floral organ defects and reduced fertility ([D] and [E]). Petal (P) length and width was reduced in 5mARF17 flowers. (F) to (H) Scanning electron micrographs of ARF17 T2 (F) and 5mARF17 T2 (G) reproductive structures. Arrowheads point to the stamen. (H) is a magnified view of 5mARF17 stamen. Note the reduced filament length, abnormal stamen orientation, and reduced anther size in 5mARF17 flowers. The 5mARF17 flowers in (G) and (H) were from infertile plants. (I) Wild-type Col-0 plant floral phyllotaxy along the primary stem. (J) 5mARF17 floral phyllotaxy along the primary and lateral stems. 5mARF17 plants have reduced fertility, illustrated by the failure to produce filled seedpods. The inset shows a magnified view of altered 5mARF17 floral phyllotaxy. (K) Mature wild-type Col-0 flower (left) and Col-0 flower with sepals and petals removed to expose reproductive organs (right). (L) Mature 35S:5mARF17 flower (left) and 35S:5mARF17 flower with sepals and petals removed to expose reproductive organs (right). (M) Average hypocotyl length, primary root length, and lateral root number (expressed as a fraction of the primary root length) of 7-d-old untransformed control seedlings (C; black bars) and 7-d-old progeny of two ARF17 T3 homozygous lines (gray bars) and two 5mARF17 T3 heterozygous lines (white bars). The 5mARF17 progeny with wild-type phenotypes (−) lacked the transgene; those with mild (+) or severe (++) phenotypes scored positive for the 5mARF17 transgene. The number of plants analyzed is indicated above each bar, and error bars represent standard deviations.
Figure 5.
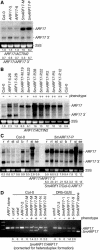
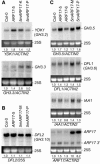
Phenotypic 5mARF17 Plants Accumulate Increased ARF17 mRNA. (A) RNA gel blot analysis of 7 μg total RNA prepared from 16-d-old T2 seedlings with a 3′ ARF17 probe. The positions of full-length ARF17 mRNA and ARF17 3′ cleavage product are noted on the right. The 25S rRNA is shown as a loading control. The blot was rehybridized with an ACTIN2 probe, and normalized values of ARF17 full-length mRNA to ACTIN2 mRNA (with Col-0 levels set at 1.0) and ratios of ARF17 full-length mRNA to 3′ cleavage product are indicated. (B) RNA gel blot analysis of 10 μg total RNA prepared from rosette leaves of 32-d-old T2 plants with a 3′ ARF17 probe. The absence (−) or presence of a mild (+) or severe (++) rosette leaf phenotype is indicated. The positions of full-length ARF17 mRNA and ARF17 3′ cleavage products are noted at the right. Normalization was as in (A). (C) RNA gel blot analysis of 5 μg total RNA prepared from Col-0 and 5mARF17 root (r), rosette leaf (rl), stem (st), cauline leaf (cl), buds and inflorescence meristems (b), flower (f), silique and embryonic tissues (si), and 12-d-old seedling (se) tissues with a 3′ ARF17 probe. The positions of full-length ARF17 mRNA and ARF17 3′ cleavage product are noted at the right. Two uncharacterized ARF17-hybridizing RNAs, one longer and one shorter than the full-length ARF17 transcript, accumulated in siliques of Col-0 and 5mARF17 plants. Ratios of ARF17 full-length mRNA to 3′ cleavage product are indicated, along with the relative levels of ARF17 mRNA in 5mARF17 plants compared with wild-type Col-0 plants in each tissue (5mARF17/Col-0 ARF17). (D) Expression of 5mARF17 mRNA in untransformed (untxf) and T2 transgenic plants that were individual progeny of the indicated primary transformant. Rosette leaf RNA was reverse transcribed, and the resulting cDNA was PCR-amplified to completion, then digested with _Apa_LI, which cuts the 5mARF17 amplicon but not the ARF17 amplicon or heteroduplex molecules resulting from annealing ARF17 and 5mARF17 strands. Agarose gel separation and ethidium bromide staining revealed the full-length PCR product (330 bp) and an _Apa_LI digestion fragment (250 bp). Each lane is an analysis of an individual plant, and the presence (+) or absence (−) of a rosette leaf phenotype in that plant is indicated. DNA gel blot analysis (data not shown) was used to quantitate the 330- and 250-bp DNAs, and the amount of 5mARF17 relative to ARF17 after correcting for heteroduplex formation is shown below each lane.
Figure 6.
IAA Treatment of Wild-Type and 5mARF17 Plants. (A) Primary root length of 8-d-old Col-0 and T2 seedlings mock treated (white bars) or treated with 50 nM IAA (black bars). 5mARF17 progeny with wild-type phenotypes (−) lacked the transgene; those with mild (+) or severe (++) phenotypes scored positive for the 5mARF17 transgene. The number of plants analyzed is indicated above each bar, and standard deviations are shown (except in cases where fewer than three plants were analyzed). (B) Hypocotyl length of 7-d-old Col-0 and T2 seedlings mock treated (white bars) or treated with 0.1 μM IAA (gray bars) and 10 μM IAA (black bars). 5mARF17 progeny with wild-type phenotypes (−) lacked the transgene; those with mild (+) or severe (++) phenotypes scored positive for the 5mARF17 transgene. The number of plants analyzed is indicated above each bar, and standard deviations are shown (except in cases where fewer than three plants were analyzed). (C) RNA gel blot analysis of 10 μg total RNA prepared from IAA treated (+) or untreated (−) Col-0 seedlings with DNA probes complementary to miR160, miR164, miR167, or U6 (loading control). Blots were stripped between each hybridization. The positions of 32P-labeled RNA oligonucleotides are noted at the right. Also shown are RNA gel blot analyses of 10 μg of the same RNA with RNA probes complementary to IAA1, IAA19, or 3′ ARF17. The positions of each full-length transcript and ARF17 3′ cleavage product are noted at the left. The 25S rRNA is shown as a loading control. Normalized values of miRNA to U6 RNA and IAA1, IAA19, and ARF17 full-length mRNAs to ACTIN2 mRNA (with untreated Col-0 levels set at 1.0) and ratios of ARF17 full-length mRNA to 3′ cleavage product are indicated.
Figure 7.
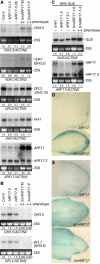
_GH3_-Like Expression in 16-d-Old 5mARF17 Seedlings. (A) RNA gel blot analysis of 7 μg of total RNA from 16-d-old seedlings with RNA probes complementary to YDK1/GH3.2 or GH3.3. The 25S rRNA is shown as a loading control. Blots were rehybridized with an ACTIN2 probe, and normalized values of _GH3_-like mRNAs to ACTIN2 mRNA are indicated, with mRNA levels in Col-0 control plants set at 1.0. (B) Similar analysis with a probe complementary to DFL2/GH3.10. Normalized values of DFL2 mRNA to 25S rRNA are indicated, with the mRNA level in Col-0 control plants set at 1.0. (C) Similar analysis with a probe complementary to GH3.5, DFL1/GH3.6, IAA1, and 3′ ARF17. Normalizations were as in (A).
Figure 8.
_GH3_-Like Gene Expression Changes in 5mARF17 Rosette Leaves. (A) RNA gel blot analysis of 7 μg of total RNA from 30-d-old rosette leaves with RNA probes complementary to GH3.3, YDK1/GH3.2, DFL2/GH3.10, IAA1, or 3′ ARF17. The absence (−) or presence of a severe (++) rosette leaf phenotype is indicated. The positions of each full-length transcript and ARF17 3′ cleavage product are noted at the right. The 25S rRNA is shown as a loading control. Normalized values of mRNAs to ACTIN2 mRNA are indicated, with mRNA levels in Col-0 control plants set at 1.0. Because YDK1 transcripts were not detected (ND) in Col-0 or ARF17 control rosette leaves, the approximately fourfold increase in YDK1 signal observed represents a minimum. (B) Similar analysis with probes complementary to GH3.5 or DFL1/GH3.6. These transcripts were not detected in 5mARF17 rosette leaves, so the fold changes observed represent a minimum. (C) RNA gel blot analysis of 7 μg of total RNA from 30-d-old rosette leaves with RNA probes complementary to GUS or 3′ ARF17. The absence (−) or presence of a mild (+) or severe (++) rosette leaf phenotype is indicated. The positions of each full-length transcript and ARF17 3′ cleavage product are noted at the right. Normalization is as in (A). (D) Fifteen-day-old ARF17 DR5-GUS and 5mARF17 DR5-GUS cotyledons. (E) Twenty-eight-day-old ARF17 DR5-GUS and 5mARF17 DR5-GUS rosette leaves with moderate (top) or more severe (bottom) leaf defects.
Similar articles
- Molecular Manipulation of the miR160/AUXIN RESPONSE FACTOR Expression Module Impacts Root Development in Arabidopsis thaliana.
Zimmerman K, Pegler JL, Oultram JMJ, Collings DA, Wang MB, Grof CPL, Eamens AL. Zimmerman K, et al. Genes (Basel). 2024 Aug 7;15(8):1042. doi: 10.3390/genes15081042. Genes (Basel). 2024. PMID: 39202402 Free PMC article. - The Arabidopsis thaliana Double-Stranded RNA Binding Proteins DRB1 and DRB2 Are Required for miR160-Mediated Responses to Exogenous Auxin.
Zimmerman K, Pegler JL, Oultram JMJ, Collings DA, Wang MB, Grof CPL, Eamens AL. Zimmerman K, et al. Genes (Basel). 2024 Dec 21;15(12):1648. doi: 10.3390/genes15121648. Genes (Basel). 2024. PMID: 39766914 Free PMC article. - MiR160 and its target genes ARF10, ARF16 and ARF17 modulate hypocotyl elongation in a light, BRZ, or PAC-dependent manner in Arabidopsis: miR160 promotes hypocotyl elongation.
Dai X, Lu Q, Wang J, Wang L, Xiang F, Liu Z. Dai X, et al. Plant Sci. 2021 Feb;303:110686. doi: 10.1016/j.plantsci.2020.110686. Epub 2020 Oct 20. Plant Sci. 2021. PMID: 33487334 - Auxin: regulation, action, and interaction.
Woodward AW, Bartel B. Woodward AW, et al. Ann Bot. 2005 Apr;95(5):707-35. doi: 10.1093/aob/mci083. Epub 2005 Mar 4. Ann Bot. 2005. PMID: 15749753 Free PMC article. Review. - Auxin regulation of Arabidopsis flower development involves members of the AINTEGUMENTA-LIKE/PLETHORA (AIL/PLT) family.
Krizek BA. Krizek BA. J Exp Bot. 2011 Jun;62(10):3311-9. doi: 10.1093/jxb/err127. Epub 2011 Apr 21. J Exp Bot. 2011. PMID: 21511900 Review.
Cited by
- Solanum lycopersicum AUXIN RESPONSE FACTOR 9 regulates cell division activity during early tomato fruit development.
de Jong M, Wolters-Arts M, Schimmel BC, Stultiens CL, de Groot PF, Powers SJ, Tikunov YM, Bovy AG, Mariani C, Vriezen WH, Rieu I. de Jong M, et al. J Exp Bot. 2015 Jun;66(11):3405-16. doi: 10.1093/jxb/erv152. Epub 2015 Apr 16. J Exp Bot. 2015. PMID: 25883382 Free PMC article. - Cleavage of INDOLE-3-ACETIC ACID INDUCIBLE28 mRNA by microRNA847 upregulates auxin signaling to modulate cell proliferation and lateral organ growth in Arabidopsis.
Wang JJ, Guo HS. Wang JJ, et al. Plant Cell. 2015 Mar;27(3):574-90. doi: 10.1105/tpc.15.00101. Epub 2015 Mar 20. Plant Cell. 2015. PMID: 25794935 Free PMC article. - STA1, an Arabidopsis pre-mRNA processing factor 6 homolog, is a new player involved in miRNA biogenesis.
Ben Chaabane S, Liu R, Chinnusamy V, Kwon Y, Park JH, Kim SY, Zhu JK, Yang SW, Lee BH. Ben Chaabane S, et al. Nucleic Acids Res. 2013 Feb 1;41(3):1984-97. doi: 10.1093/nar/gks1309. Epub 2012 Dec 24. Nucleic Acids Res. 2013. PMID: 23268445 Free PMC article. - The Integrated mRNA and miRNA Approach Reveals Potential Regulators of Flowering Time in Arundina graminifolia.
Ahmad S, Lu C, Gao J, Wei Y, Xie Q, Jin J, Zhu G, Yang F. Ahmad S, et al. Int J Mol Sci. 2023 Jan 15;24(2):1699. doi: 10.3390/ijms24021699. Int J Mol Sci. 2023. PMID: 36675213 Free PMC article.
References
- Achard, P., Herr, A., Baulcombe, D.C., and Harberd, N.P. (2004). Modulation of floral development by a gibberellin-regulated microRNA. Development 131, 3357–3365. - PubMed
- Aida, M., Vernoux, T., Furutani, M., Traas, J., and Tasaka, M. (2002). Roles of PIN-FORMED1 and MONOPTEROS in pattern formation of the apical region of the Arabidopsis embryo. Development 129, 3965–3974. - PubMed
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301, 653–657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 GM071200/GM/NIGMS NIH HHS/United States
- R24 GM069512/GM/NIGMS NIH HHS/United States
- F32-GM071200/GM/NIGMS NIH HHS/United States
- R24-GM069512/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials