Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist - PubMed (original) (raw)
Comparative Study
. 2005 Apr 18;201(8):1307-18.
doi: 10.1084/jem.20041385.
Christie M Orschell, D Wade Clapp, Giao Hangoc, Scott Cooper, P Artur Plett, W Conrad Liles, Xiaxin Li, Barbara Graham-Evans, Timothy B Campbell, Gary Calandra, Gary Bridger, David C Dale, Edward F Srour
Affiliations
- PMID: 15837815
- PMCID: PMC2213145
- DOI: 10.1084/jem.20041385
Comparative Study
Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist
Hal E Broxmeyer et al. J Exp Med. 2005.
Abstract
Improving approaches for hematopoietic stem cell (HSC) and hematopoietic progenitor cell (HPC) mobilization is clinically important because increased numbers of these cells are needed for enhanced transplantation. Chemokine stromal cell derived factor-1 (also known as CXCL12) is believed to be involved in retention of HSCs and HPCs in bone marrow. AMD3100, a selective antagonist of CXCL12 that binds to its receptor, CXCR4, was evaluated in murine and human systems for mobilizing capacity, alone and in combination with granulocyte colony-stimulating factor (G-CSF). AMD3100 induced rapid mobilization of mouse and human HPCs and synergistically augmented G-CSF-induced mobilization of HPCs. AMD3100 also mobilized murine long-term repopulating (LTR) cells that engrafted primary and secondary lethally-irradiated mice, and human CD34(+) cells that can repopulate nonobese diabetic-severe combined immunodeficiency (SCID) mice. AMD3100 synergized with G-CSF to mobilize murine LTR cells and human SCID repopulating cells (SRCs). Human CD34(+) cells isolated after treatment with G-CSF plus AMD3100 expressed a phenotype that was characteristic of highly engrafting mouse HSCs. Synergy of AMD3100 and G-CSF in mobilization was due to enhanced numbers and perhaps other characteristics of the mobilized cells. These results support the hypothesis that the CXCL12-CXCR4 axis is involved in marrow retention of HSCs and HPCs, and demonstrate the clinical potential of AMD3100 for HSC mobilization.
Figures
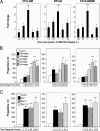
Figure 1.
Time and dose response effects of AMD3100 as a mobilizer of HPC (CFU-GM, BFU-E and CFU-GEMM) to the blood of mice. (A) Time course of mobilization in response to a single s.c. injection of 5 mg/kg AMD3100 into C3H/HeJ mice; results are for one full experiment (5 mice/group and time 0 control numbers of CFU-GM, BFU-E, and CFU-GEMM per ml of blood were 78 ± 18, 17 ± 2, and 10 ± 2, respectively). (B) Dose response analysis at 1 h post s.c. injections of 0.625, 1.25, 2.5, 5.0, or 10 mg/kg AMD3100 into C3H/HeJ mice; results are average of 7 mice/group from a total of two separate experiments. (C) Influence of multiple injections of 5 mg/kg AMD3100 or saline given on day 1; days 1 and 2; or days 1, 2, and 3 given 24 h apart, and analyzed 1 h after the last injection into C3H/HeJ mice; results are average of 14 mice for control group for different timed saline injections and 8 mice for each test group from a total of two complete experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 2.
Comparative AMD3100 enhancement of G-CSF–induced mobilization of CFU-GM, BFU-E, and CFU-GEMM in: (A) 2 d G-CSF: C57Bl/6, C3H/HeJ, DBA/2, (B) 4 d G-CSF: C57Bl/6, and (C) 4 d of G-CSF: Fancc−/− and +/+ mice. The results shown in A are an average from a total of three experiments of 15 C57Bl/6, 15 DBA/2, and 11 C3H/HeJ mice. Results in B are for one experiment of 5 mice/group. Results shown in C are the averages of 15 wild-type and 8 Fancc−/− mice per group averaged from a total of two experiments. For parts A–C, all experimental points within a group are at least P < 0.001 compared with the control for the specific progenitor cell of each mouse strain.
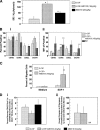
Figure 3.
AMD3100 mobilizes competitive repopulating mouse HSCs with self-renewal capacity (A) and G-CSF synergizes this effect (B). (A, left) 18 C57Bl/6 mice (CD45.2) serving as donors were injected s.c. with 0.1 ml saline, and 36 C57Bl/6 mice were injected with 0.1 ml AMD3100 at 5 mg/kg (=∼100 μg/mouse). Blood was collected 1 h later and LDMNCs isolated. BM from nonirradiated B6.BoyJ (CD45.1) mice served as competitor cells. The ratio of donor (CD45.2) blood cells to competitor (CD45.1) BM cells was set as the number of LDMNC blood cells in 3 donor mice to a constant number of 0.5 × 106 competitor BM cells equaling 3:1. A 2:1 ratio was the number of LDMNCs in blood of 2 donor mice to 0.5 × 106 competitor cells, and a 1:1 ratio was LDMNC in 1 donor mouse to 0.5 × 106 competitor cells. Results are based on analysis of transplantation of 6 lethally irradiated recipients per test group of i.v. infused cells. Right panel: BM cells were removed from femurs of primary mice 4 mo after injection of 3:1 saline or 3:1 AMD3100:competitor cell mixture and 2.5 × 106 marrow cells from 3 mice of each group injected separately into 3 lethally secondary irradiated B6.BoyJ mice in a noncompetitive assay. Results are shown as percent CD45.2 C57Bl/6 donor cell chimerism in CD45.1 B6.BoyJ recipients. *P < 0.05; **P < 0.01; ***P < 0.001 compared with group 1 at each time point. (B) G-CSF and AMD3100 were administered as in Figure 2, part A, with G-CSF given twice a day for 2 d. Left panel: competitive repopulation in primary recipients 6 mo post transplant. The results are an average of mouse cells from five donors into six recipients each. Right panel: secondary repopulation in a noncompetitive assay 6 mo after transplant. Results are an average of 3 donor mice with cells transplanted into three recipients each. *P < 0.01 or **P < 0.001 compared with control.
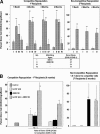
Figure 4.
Influence of AMD3100, alone and with G-CSF, on circulating HPCs in healthy human volunteers. (A) Influence of multiple injections of AMD3100 alone. Three volunteers were injected i.v. with AMD3100 (80 μg/kg) on the first day (day 1) and again at 24 h. Progenitors/ml blood were assessed preinjection (day 1, 0 h) and at the noted time intervals. Fold changes are calculated based on the following day 1, 0 h progenitors/ml for three donors: CFU-GM (387, 78, 201), BFU-E (712, 78, 217), and CFU-GEMM (77, 29, 93) grown in methylcellulose cultures in the presence of Epo, stem cell factor, and interleukin-3. The fold changes for CFU-GM also include progenitor cells/ml as calculated in agar culture medium with GM-CSF and stem cell factor (control numbers for three donors: 108, 18, 54). *P < 0.001 compared with 0 h counts of that particular day. (B) Influence of AMD3100 on mobilization of circulating HPCs in healthy human volunteers receiving G-CSF or G-CSF plus AMD3100 (160 μg/kg). The results shown are the mean plus range of fold increases of the absolute numbers of CFU-GM and CFU-GEMM per ml of blood for two healthy volunteers each without apheresis. Fold changes are based on the following numbers of G-CSF day 5, 0 h control progenitor cells/ml: group I: G-CSF (CFU-GM: 34403 and 27194; CFU-GEMM: 14363 and 10083); group II: G-CSF (4 d) plus AMD3100 (day 5; CFU-GM: 11968 and 2675; CFU-GEMM: 3366 and 510), and group III: G-CSF (5 d) plus AMD3100 (day 5; CFU-GM: 2698 and 2380; CFU-GEMM: 1028 and 1238). (C) Results of apheresis after mobilization of circulating HPCs. Results are expressed as total progenitors collected (in thousands) for donors receiving G-CSF (n = 3), G-CSF + AMD3100 (160 μg/kg; n = 3), or AMD3100 (240 μg/kg; n = 4). *P < 0.01 for G-CSF– or AMD3100 versus G-CSF + AMD3100-mobilized cells. (D) Results of apheresis shown in C but expressed as progenitors mobilized per kg donor. *P < 0.05 for same comparisons as in C.
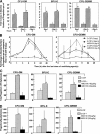
Figure 5.
Influence of AMD3100, G-CSF, and the combination of G-CSF plus AMD3100 on mobilization of NOD-SCID SRCs from normal human volunteers (apheresis samples), surface expression of adhesion molecules and chemotaxis of CD34**+** cells, and homing of mobilized murine Sca1**+Lin−** cells. (A) SRCs per kg in apheresis samples from G-CSF– (n = 3), G-CSF + AMD3100- (160 μg/kg; n = 3), and AMD3100- (240 μg/kg; n = 4) mobilized circulating blood. Each set of test samples was assayed simultaneously in limiting dilutions in conditioned NOD-SCID mice. For every sample, four different cell concentrations were used and four mice were transplanted with each cell concentration. Mice were assayed for chimerism 8 wk later; those that demonstrated >0.2% chimerism (total CD45+ cells in BM) were considered to be positive. Percentage of negative mice were used to calculate SRC frequencies. (B, i) Expression of CD49d (VLA-4), CD49e (VLA-5), CD26L (L-selectin), and CXCR4 on mobilized CD34+ cells (mean ± 1SEM of percent positive cells of five to seven different G-CSF samples, three AMD3100 plus G-CSF samples, and four BM samples). (B, ii) Mean fluorescent intensity (MFI) of positive samples. Same number of samples evaluated as in B, i except G-CSF group has a different number (n =7). (C) CD34+ cells isolated from G-CSF (n = 6) and G-CSF plus AMD3100 (n = 3) mobilized peripheral blood were assessed for chemotaxis to SDF-1/CXCL12 (100 ng/ml) and results expressed as percentage of migrated cells. Data for each sample were collected in duplicate. (D, i) Homing of CD45.2+ Sca1+Lin− BM cells from C57Bl/6 mice into lethally irradiated CD45.1+ B6.BoyJ BM from samples mobilized with G-CSF (2 times/d for 4 d as in Fig. 2B; n = 3), G-CSF + AMD3100 (5 mg/kg; n = 4), and AMD3100 (5 mg/kg; n = 3). Homing was assessed as in Materials and methods. (D, ii) Competitive repopulation of CD45.2+ BM cells recovered after homing shown in D, i. Results of chimerism are after 4 mo in 2° irradiated B6.BoyJ recipients. For A–D, significant differences compared with G-CSF: *P < 0.001; **P < 0.01; ***P < 0.05; all other values are P > 0.05. NA, data not depicted.
Similar articles
- AMD3100 and CD26 modulate mobilization, engraftment, and survival of hematopoietic stem and progenitor cells mediated by the SDF-1/CXCL12-CXCR4 axis.
Broxmeyer HE, Hangoc G, Cooper S, Campbell T, Ito S, Mantel C. Broxmeyer HE, et al. Ann N Y Acad Sci. 2007 Jun;1106:1-19. doi: 10.1196/annals.1392.013. Epub 2007 Mar 14. Ann N Y Acad Sci. 2007. PMID: 17360804 - AMD3100 mobilizes hematopoietic stem cells with long-term repopulating capacity in nonhuman primates.
Larochelle A, Krouse A, Metzger M, Orlic D, Donahue RE, Fricker S, Bridger G, Dunbar CE, Hematti P. Larochelle A, et al. Blood. 2006 May 1;107(9):3772-8. doi: 10.1182/blood-2005-09-3592. Epub 2006 Jan 26. Blood. 2006. PMID: 16439684 Free PMC article. - Effective mobilization of hematopoietic progenitor cells in G-CSF mobilization defective CD26-/- mice through AMD3100-induced disruption of the CXCL12-CXCR4 axis.
Paganessi LA, Walker AL, Tan LL, Holmes I, Rich E, Fung HC, Christopherson KW 2nd. Paganessi LA, et al. Exp Hematol. 2011 Mar;39(3):384-90. doi: 10.1016/j.exphem.2010.12.003. Epub 2010 Dec 17. Exp Hematol. 2011. PMID: 21168468 Free PMC article. - Role of CXCR4 chemokine receptor blockade using AMD3100 for mobilization of autologous hematopoietic progenitor cells.
Flomenberg N, DiPersio J, Calandra G. Flomenberg N, et al. Acta Haematol. 2005;114(4):198-205. doi: 10.1159/000088410. Acta Haematol. 2005. PMID: 16269859 Review. - Recent advances on the use of the CXCR4 antagonist plerixafor (AMD3100, Mozobil™) and potential of other CXCR4 antagonists as stem cell mobilizers.
De Clercq E. De Clercq E. Pharmacol Ther. 2010 Dec;128(3):509-18. doi: 10.1016/j.pharmthera.2010.08.009. Epub 2010 Sep 15. Pharmacol Ther. 2010. PMID: 20826182 Review.
Cited by
- The chemokine system in innate immunity.
Sokol CL, Luster AD. Sokol CL, et al. Cold Spring Harb Perspect Biol. 2015 Jan 29;7(5):a016303. doi: 10.1101/cshperspect.a016303. Cold Spring Harb Perspect Biol. 2015. PMID: 25635046 Free PMC article. Review. - Increased yield of endothelial cells from peripheral blood for cell therapies and tissue engineering.
Jamiolkowski RM, Kang SD, Rodriguez AK, Haseltine JM, Galinat LJ, Jantzen AE, Carlon TA, Darrabie MD, Arciniegas AJ, Mantilla JG, Haley NR, Noviani M, Allen JD, Stabler TV, Frederiksen JW, Alzate O, Keil LG, Liu S, Lin FH, Truskey GA, Achneck HE. Jamiolkowski RM, et al. Regen Med. 2015 May;10(4):447-60. doi: 10.2217/rme.15.2. Regen Med. 2015. PMID: 26022764 Free PMC article. - Mobilization of hematopoietic stem/progenitor cells: general principles and molecular mechanisms.
Bonig H, Papayannopoulou T. Bonig H, et al. Methods Mol Biol. 2012;904:1-14. doi: 10.1007/978-1-61779-943-3_1. Methods Mol Biol. 2012. PMID: 22890918 Free PMC article. Review. - SHIPi Enhances Autologous and Allogeneic Hematolymphoid Stem Cell Transplantation.
Fernandes S, Brooks R, Gumbleton M, Park MY, Russo CM, Howard KT, Chisholm JD, Kerr WG. Fernandes S, et al. EBioMedicine. 2015 Mar 1;2(3):205-213. doi: 10.1016/j.ebiom.2015.02.004. EBioMedicine. 2015. PMID: 26052545 Free PMC article. - Molecular mechanisms underlying adhesion and migration of hematopoietic stem cells.
Sahin AO, Buitenhuis M. Sahin AO, et al. Cell Adh Migr. 2012 Jan-Feb;6(1):39-48. doi: 10.4161/cam.18975. Cell Adh Migr. 2012. PMID: 22647939 Free PMC article. Review.
References
- Kondo, M., A.J. Wagers, M.G. Manz, S.S. Prohaska, D.C. Scherer, G.F. Beilhack, J.A. Shizuru, and I.L. Weissman. 2003. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu. Rev. Immunol. 21:759–806. - PubMed
- Manz, M.G., K. Akashi, and I.L. Weissman. 2004. Biology of hematopoietic stem and progenitor cells. Thomas' Hematopoietic Cell Transplantation. 3rd ed. K.G. Blume, S.J. Forman, and F.R. Appelbaum, editors. Blackwell Publishing Ltd., Malden, MA. 69–95.
- Cooper, S., and H.E. Broxmeyer. 1996. Measurement of interleukin-3 and other hematopoietic growth factors, such as GM-CSF, G-CSF, M-CSF, erythropoietin and the potent co-stimulating cytokines steel factor and Flt-3 ligand. Current Protocols in Immunology. J.E. Coligan, A.M. Kruisbeek, D.H. Margulies, E.M. Shevach, W. Strober, and R. Coico, editors. John Wiley & Sons, Inc., NY. Supp. 18, 6.4.1–6.4.12.
- Wright, D.E., A.J. Wagers, A.P. Gulati, F.L. Johnson, and L.L. Weissman. 2001. Physiological migration of hematopoietic stem and progenitor cells. Science. 294:1933–1936. - PubMed
- Abkowitz, J.L., A.E. Robinson, S. Kale, M.W. Long, and J. Chen. 2003. The mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood. 102:1249–1253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL67384/HL/NHLBI NIH HHS/United States
- P01 HL053586/HL/NHLBI NIH HHS/United States
- R01 DK053674/DK/NIDDK NIH HHS/United States
- R01 DK53674/DK/NIDDK NIH HHS/United States
- P01 HL53586/HL/NHLBI NIH HHS/United States
- T32 DK07519/DK/NIDDK NIH HHS/United States
- R01 DK53574/DK/NIDDK NIH HHS/United States
- R01 HL55716/HL/NHLBI NIH HHS/United States
- R01 HL067384/HL/NHLBI NIH HHS/United States
- R01 HL63219/HL/NHLBI NIH HHS/United States
- P50 DK049218/DK/NIDDK NIH HHS/United States
- T32 DK007519/DK/NIDDK NIH HHS/United States
- P50 DK49218/DK/NIDDK NIH HHS/United States
- R01 HL055716/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical