Systemic lupus erythematosus serum IgG increases CREM binding to the IL-2 promoter and suppresses IL-2 production through CaMKIV - PubMed (original) (raw)
Systemic lupus erythematosus serum IgG increases CREM binding to the IL-2 promoter and suppresses IL-2 production through CaMKIV
Yuang-Taung Juang et al. J Clin Invest. 2005 Apr.
Abstract
Systemic lupus erythematosus (SLE) T cells express high levels of cAMP response element modulator (CREM) that binds to the IL-2 promoter and represses the transcription of the IL-2 gene. This study was designed to identify pathways that lead to increased binding of CREM to the IL-2 promoter in SLE T cells. Ca(2+)/calmodulin-dependent kinase IV (CaMKIV) was found to be increased in the nucleus of SLE T cells and to be involved in the overexpression of CREM and its binding to the IL-2 promoter. Treatment of normal T cells with SLE serum resulted in increased expression of CREM protein, increased binding of CREM to the IL-2 promoter, and decreased IL-2 promoter activity and IL-2 production. This process was abolished when a dominant inactive form of CaMKIV was expressed in normal T cells. The effect of SLE serum resided within the IgG fraction and was specifically attributed to anti-TCR/CD3 autoantibodies. This study identifies CaMKIV as being responsible for the increased expression of CREM and the decreased production of IL-2 in SLE T cells and demonstrates that anti-TCR/CD3 antibodies present in SLE sera can account for the increased expression of CREM and the suppression of IL-2 production.
Figures
Figure 1
Characterization of a new anti–human CREM-specific antibody. (A) The new anti-CREM antibody recognizes CREM but not CREB and ATF-1. Nuclear proteins from primary T cells were resolved in SDS gel and then subjected to Western blotting with the new CREM antibody. The membrane was then stripped and blotted sequentially with anti–ATF-1 and anti-CREB antibody. The anti-CREM antibody recognizes only 1 band at the site expected for CREMα. Similarly, the anti–ATF-1 and anti-CREB antibodies recognize bands at the expected sites. (B) The new anti-CREM antibody disrupts the formation of –180 site–defined oligonucleotide/protein complex. Nuclear proteins from Jurkat T cells were incubated with the –180 oligonucleotide and 1 μl of serially diluted preimmune or immune serum. (C) Human CREM–defined peptide (amino acids 21–34), but not hnRNP-defined peptide (amino acids 263–275), blocks the ability of anti-CREM antibody to disrupt the formation of –180 site–defined oligonucleotide/protein complex. Anti-CREM serum was used at 10–3 dilution in the presence or absence of 1 μM of the indicated peptide.
Figure 2
Increased binding of CREM to the –180 site of the IL-2 promoter in SLE T cells. (A) Nuclear proteins were incubated with a 32P-labeled oligonucleotide encoding the –180 site of the IL-2 promoter as described in Methods. Shown are 3 independently conducted EMSAs, each containing 1 pair of simultaneously purified normal (N) and SLE T cells. (B) Nuclear proteins from an SLE patient were incubated with the –180 oligonucleotide with or without antibody against CREB, CREM, or c-Jun for 15 minutes, followed by shift analysis. (C) Nuclear proteins from primary T cells were incubated with 32P-labeled oligonucleotide encoding the –180 site of the IL-2 promoter. Where indicated, unlabeled wild-type or mutant oligonucleotides containing 2 mutated nucleotides were also added into the reaction.
Figure 3
SLE sera stimulate the expression of CREM and its binding to the –180 element of IL-2 promoter. (A) SLE sera induce CREM mRNA. Normal T cells were stimulated with normal, RA, or SLE sera for 2 hours before RNA was analyzed for the expression level of CREM and actin. (B) Cumulative analysis of the effect of SLE sera on the expression of CREM mRNA. The y axis represents the ratio between the mRNA expression levels of CREM and those of actin in normal T cells treated with SLE, RA, or normal serum. (C) SLE sera induce the expression of CREM in the nucleus. Normal T cells were cultured for 2 hours in RPMI containing 1% serum from normal, RA, or SLE patients. Nuclear proteins were harvested and assayed using Western blotting with the indicated antibodies. Shown are 2 representative Western blots, each containing simultaneously purified nuclear proteins from normal T cells treated with normal, RA, or SLE serum. (D) Cumulative effect of SLE sera on the expression of nuclear CREM protein in normal T cells. The y axis represents the ratio of the expression levels of CREM to those of CREB protein in normal T cells treated with normal, RA, or SLE sera. (E) Increased formation of –180 complex in normal T cells treated with SLE sera. Nuclear proteins from normal T cells treated with sera from normal, RA, or SLE patients were analyzed by EMSA to measure their binding activity to oligonucleotides encoding the –180 element. (F) The y axis represents the –180/protein complex intensity in the T cells treated with SLE, RA, or normal serum. *P < 0.05.
Figure 4
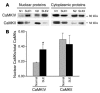
Increased expression of CaMKIV in the nucleus of SLE T cells. (A) Both cytoplasmic and nuclear proteins were isolated from normal and SLE T cells. Western blots were then conducted by sequential blotting of the membrane with antibody against CaMKIV or CaMKII. Representative blots from 2 patient-control pairs are shown. (B) Eleven pairs were studied. Nuclear densitometric readings for CaMKIV, but not CaMKII, are higher (P < 0.01) in SLE T cells than in normal T cells. *P < 0.05.
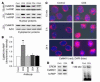
Figure 5
CaMKIV localization in normal and SLE T cells. Primary T cells from either normal or human SLE patients were stained with Cyanin 3–conjugated anti-CaMKIV or DAPI, and the images were merged as indicated. Five hundred cells were evaluated from each sample. Magnification, ×1000.
Figure 6
Overexpression of CaMKIV(i), but not overexpression of CaMKII(i), abolishes the SLE serum–induced –180 site/protein complex formation. (A) Normal T cells were transfected with control plasmid or plasmids encoding catalytically inactive CaMKII or CaMKIV [CaMKII(i) or CaMKIV(i)]. Three hours later, cells were treated with SLE, RA, or normal sera for 2 more hours and then harvested for EMSA analysis. (B) Cumulative data from 5 experiments are presented. (C) CaMKII(i) does not abolish the effect of SLE serum on the formation of the –180/protein complex. (D) Cumulative data are presented. *P < 0.05.
Figure 7
CaMKIV upregulates the expression of CREM and its binding to the –180 site of the IL-2 promoter in SLE T cells. (A) Normal T cells were transfected with a CaMKIV expression construct for the indicated time, and the lysates were blotted with an anti-CaMKIV or an anti-hnRNP (control) antibody. (B) SLE T cells were transfected with control plasmid or a plasmid overexpressing CaMKIV and then were treated with PMA and ionomycin. Four hours later, nuclear proteins were purified, and Western blotting was conducted by sequential use of antibodies as indicated. An antibody against hnRNP was used as control. (C) SLE T cells were transfected with control plasmid or a plasmid expressing wild-type CaMKIV and then were treated with PMA and ionomycin. At the indicated time points, cells were harvested, and EMSA was conducted using the oligonucleotide encoding the –180 site of the IL-2 promoter. (D) Cumulative time-curve results of the effect of CaMKIV overexpression in normal and SLE T cells. The y axis represents the ratio of the intensity of the –180/protein complex in the CaMKIV-transfected cells to that in the cells transfected with control plasmid. Normal and SLE T cells were subjected to the experimental protocol detailed in C. Filled symbols, SLE T cells; open symbols, normal T cells. *P < 0.05.
Figure 8
CaMKIV suppresses the –180 site–driven reporter activity in SLE T cells. Freshly purified normal and SLE T cells were transiently cotransfected with the luciferase reporter construct driven by the –180 site of the IL-2 promoter (2 copies placed in tandem) and with control plasmid or a plasmid expressing wild-type CaMKIV, and then stimulated with PMA and ionomycin. The y axis represents the β-gal–normalized luciferase activity, which was assayed 6 hours after transfection. *P < 0.05.
Figure 9
SLE sera enhance CaMKIV-mediated activation of the –180/protein complex in normal T cells. (A) SLE sera augment the effect of CaMKIV on the formation of the –180/protein complex in normal T cells. Normal T cells were primed with normal or SLE sera for 2 hours; cells were then transfected with CaMKIV expression plasmids and treated with PMA and ionomycin. Three hours later, cells were harvested, and EMSA was performed by incubation of nuclear proteins with the –180/protein complex. (B) Cumulative results from 4 experiments (P < 0.05).
Figure 10
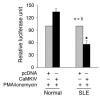
SLE sera suppress IL-2 production by normal T cells. (A) Normal T cells were incubated for 16 hours with 1% SLE serum and then cotransfected with a CaMKIV expression vector and a proximal (–575 to +57) IL-2 promoter reporter construct. Three hours later, cells were treated with PMA and ionomycin, and after 5 hours the luciferase activity was measured and normalized against β-gal activity to control for transfection efficiency. (B) Cells were subjected to the same treatment, and the produced IL-2 was determined using an ELISA.
Figure 11
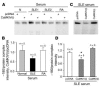
SLE serum IgG promotes the binding of protein to the –180 site of the IL-2 promoter and decreases the production of IL-2 in normal T cells. (A) SLE and normal sera were fractionated (see Methods) and subjected to electrophoresis. (B) Exposure of normal T cells to SLE sera and IgG fractions (2 μg/ml) from SLE sera increases the formation of –180/protein complex. Data from 1 experiment (n = 4) are shown. (C) SLE sera and IgG fractions of SLE sera (n = 4) limit the ability of normal T cells to produce IL-2. IL-2 production in the presence of normal sample was set at 1 (gray bars).
Figure 12
Anti-CD3 antibody activates CaMKIV and induces CREM binding to the –180 site of the IL-2 promoter in normal T cells. (A) Normal T cells were treated with either control antibody (Cont.; normal murine IgG, 1:100) or antibodies against CD3 (1:100), CD28 (1:200), CD4 (1:200), or CD45 (1:200) for the indicated time period. Nuclear and cytoplasmic proteins were blotted with antibody against CaMKIV, hnRNP, or actin. (B) Cumulative data from 4 experiments. **P < 0.01. (C) Normal T cells treated as in A were fixed and stained with anti-CaMKIV antibody or DAPI. Shown are the merged pictures after cells were stained with antibodies against CaMKIV and DAPI (which defines the location of the nucleus). Magnification, ×1000. (D) Nuclear proteins isolated from T cells treated with anti-CD3 antibody for 3 hours were blotted sequentially with antibodies against CaMKIV, CREM, and hnRNP. (E) Nuclear proteins isolated from T cells treated with anti-CD3 antibody for 3 hours were incubated with 32P-labeled –180 site oligonucleotide.
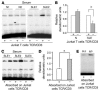
Figure 13
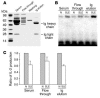
The TCR/CD3 complex represents the ligand for SLE serum IgG that is responsible for the increased expression of –180/protein complex formation. (A) Wild-type Jurkat T cells or J.EMS.T3.3 cells (a Jurkat T cell subline missing the TCR/CD3 complex) were treated with 1% normal or SLE sera for 3 hours, and nuclear extracts were subjected to a shift assay using the –180 oligonucleotide. (B) Cumulative data (n = 4) are shown. The y axis indicates relative densitometric units with the effect on TCR/CD3–positive cells set at 1. *P < 0.05. (C) Normal or SLE sera were adsorbed for 30 minutes at room temperature and another 30 minutes at 4–C on either Jurkat or J.EMS.T3.3 T cells and used to treat normal T cells for 3 hours. Nuclear extracts were subjected to a shift assay using the –180 oligonucleotide. (D) Cumulative data showing the effect of adsorption of SLE sera (n = 4) on TCR/CD3–positive and –negative Jurkat T cells. The y axis indicates relative densitometric units with the effect of sera adsorbed on TCR/CD3–positive cells set at 1. *P < 0.05. (E) SLE sera were adsorbed on TCR/CD3–positive and –negative cells, and the IgG fraction was isolated and used to treat normal T cells for 3 hours. Nuclear extracts were isolated and subjected to a shift assay using the –180 oligonucleotide. One of 2 similar experiments is shown.
Comment in
- Altered regulation of IL-2 production in systemic lupus erythematosus: an evolving paradigm.
Kammer GM. Kammer GM. J Clin Invest. 2005 Apr;115(4):836-40. doi: 10.1172/JCI24791. J Clin Invest. 2005. PMID: 15841173 Free PMC article.
Similar articles
- Antisense cyclic adenosine 5'-monophosphate response element modulator up-regulates IL-2 in T cells from patients with systemic lupus erythematosus.
Tenbrock K, Juang YT, Gourley MF, Nambiar MP, Tsokos GC. Tenbrock K, et al. J Immunol. 2002 Oct 15;169(8):4147-52. doi: 10.4049/jimmunol.169.8.4147. J Immunol. 2002. PMID: 12370343 - Molecular basis of deficient IL-2 production in T cells from patients with systemic lupus erythematosus.
Solomou EE, Juang YT, Gourley MF, Kammer GM, Tsokos GC. Solomou EE, et al. J Immunol. 2001 Mar 15;166(6):4216-22. doi: 10.4049/jimmunol.166.6.4216. J Immunol. 2001. PMID: 11238674 - Altered regulation of IL-2 production in systemic lupus erythematosus: an evolving paradigm.
Kammer GM. Kammer GM. J Clin Invest. 2005 Apr;115(4):836-40. doi: 10.1172/JCI24791. J Clin Invest. 2005. PMID: 15841173 Free PMC article. - [Interleukin-2 signaling pathway regulating molecules in systemic lupus erythematosus].
Guo Q, Chen XY, Su Y. Guo Q, et al. Beijing Da Xue Xue Bao Yi Xue Ban. 2016 Dec 18;48(6):1100-1104. Beijing Da Xue Xue Bao Yi Xue Ban. 2016. PMID: 27987522 Review. Chinese. - Role of CREM in systemic lupus erythematosus.
Xu WD, Zhang YJ, Wang W, Li R, Pan HF, Ye DQ. Xu WD, et al. Cell Immunol. 2012 Mar-Apr;276(1-2):10-5. doi: 10.1016/j.cellimm.2012.04.008. Epub 2012 Apr 19. Cell Immunol. 2012. PMID: 22560675 Review.
Cited by
- Low-Dose IL-2 in the Treatment of Lupus.
Mizui M, Tsokos GC. Mizui M, et al. Curr Rheumatol Rep. 2016 Nov;18(11):68. doi: 10.1007/s11926-016-0617-5. Curr Rheumatol Rep. 2016. PMID: 27734211 Review. - Dysregulated neutrophilic cell death in SLE: a spotlight on ferroptosis.
Ohl K, Rauen T, Tenbrock K. Ohl K, et al. Signal Transduct Target Ther. 2021 Nov 11;6(1):392. doi: 10.1038/s41392-021-00804-z. Signal Transduct Target Ther. 2021. PMID: 34764247 Free PMC article. No abstract available. - Calcium/Calmodulin-Dependent Protein Kinase IV (CaMKIV) Mediates Acute Skeletal Muscle Inflammatory Response.
Shi D, Gu R, Song Y, Ding M, Huang T, Guo M, Xiao J, Huang W, Liao H. Shi D, et al. Inflammation. 2018 Feb;41(1):199-212. doi: 10.1007/s10753-017-0678-2. Inflammation. 2018. PMID: 28971270 - SLE: translating lessons from model systems to human disease.
Singh RR. Singh RR. Trends Immunol. 2005 Nov;26(11):572-9. doi: 10.1016/j.it.2005.08.013. Epub 2005 Sep 9. Trends Immunol. 2005. PMID: 16153890 Free PMC article. Review.
References
- Linker-Israeli M, et al. Defective production of interleukin 1 and interleukin 2 in patients with systemic lupus erythematosus (SLE) J. Immunol. 1983;130:2651–2655. - PubMed
- Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv. Immunol. 1985;37:269–390. - PubMed
- Iliopoulos AG, Tsokos GC. Immuno-pathogenesis and spectrum of infections in systemic lupus erythematosus. Semin. Arthritis Rheum. 1996;25:318–336. - PubMed
- Kovacs B, Vassilopoulos D, Vogelgesang SA, Tsokos GC. Defective CD3-mediated cell death in activated T cells from patients with systemic lupus erythematosus: role of decreased intracellular TNF-alpha. Clin. Immunol. Immunopathol. 1996;81:293–302. - PubMed
- Solomou EE, Juang YT, Gourley MF, Kammer GM, Tsokos GC. Molecular basis of deficient IL-2 production in T cells from patients with systemic lupus erythematosus. J. Immunol. 2001;166:4216–4222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI042269/AI/NIAID NIH HHS/United States
- R01 AI049954/AI/NIAID NIH HHS/United States
- R01 AI49954/AI/NIAID NIH HHS/United States
- R01 AI42269/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous