Genetic analysis of lysosomal trafficking in Caenorhabditis elegans - PubMed (original) (raw)
Genetic analysis of lysosomal trafficking in Caenorhabditis elegans
Greg J Hermann et al. Mol Biol Cell. 2005 Jul.
Abstract
The intestinal cells of Caenorhabditis elegans embryos contain prominent, birefringent gut granules that we show are lysosome-related organelles. Gut granules are labeled by lysosomal markers, and their formation is disrupted in embryos depleted of AP-3 subunits, VPS-16, and VPS-41. We define a class of gut granule loss (glo) mutants that are defective in gut granule biogenesis. We show that the glo-1 gene encodes a predicted Rab GTPase that localizes to lysosome-related gut granules in the intestine and that glo-4 encodes a possible GLO-1 guanine nucleotide exchange factor. These and other glo genes are homologous to genes implicated in the biogenesis of specialized, lysosome-related organelles such as melanosomes in mammals and pigment granules in Drosophila. The glo mutants thus provide a simple model system for the analysis of lysosome-related organelle biogenesis in animal cells.
Figures
Figure 1.
Birefringent gut granules are located within acidic compartments. Birefringent gut granules (B) in a wild-type 1.5-fold stage embryo colocalized with acidified, acridine orange-stained (C) compartments (white arrows). Some acidic intestinal compartments stained by acridine orange did not contain detectable birefringent material (white arrowheads). In A–C, a black arrowhead marks the anterior of the intestinal primordium. A newly hatched L1-stage larvae (D–G) contained birefringent gut granules within acidified and autofluorescent intestinal organelles. Birefringent gut granules colocalized with autofluorescent and acidic LysoTracker Red-stained compartments (white arrows). Some acidic intestinal compartments stained by LysoTracker Red colocalized with autofluorescent, but not birefringent, gut granules (white arrowheads). In
D–
G, the intestinal lumen is marked with a black arrow.
Figure 2.
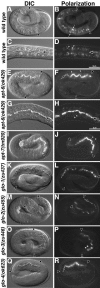
Birefringent gut granule mislocalization in Glo mutants. Wild-type pretzel-stage embryos (A and B) and L1-stage larvae (C and D) contained birefringent gut granules (white arrows in B and white arrowheads in D) within intestinal cells. The intestinal lumen is marked with a black arrow in C and D. apt-6 (E and F) pretzel-stage embryos contained gut granules within the intestinal lumen (black arrow) and a reduced number of gut granules (white arrowheads) within intestinal cells. (G and H) The presence of birefringent material within the intestinal lumen (black arrow) and intestinal cells (white arrowheads) is shown at higher magnification in a newly hatched apt-6 larva. apt-7 (I and J) pretzel-stage embryos contained gut granules within the intestinal lumen (black arrow) and a reduced number of gut granules (white arrowheads) within intestinal cells. glo-1 (K and L), glo-2 (M and N), and glo-4 (Q and R) pretzel-stage embryos lacked gut granules within the intestinal cell cytoplasm and instead gut granules (black arrows) were localized in the intestinal lumen. glo-3 (O and P) pretzel stage embryos mislocalized birefringent granules to the intestinal lumen (black arrow) and typically contained between one and five birefringent gut granules (white arrowhead) within intestinal cells. In A, B, E, and F, and I-R intestinal cells are located between the black arrowheads. C. elegans embryos are ∼50 μm in length.
Figure 3.
Analysis of autofluorescent and acidified gut granules in young adult-stage Glo mutants. (A–C) Wild-type animals contained numerous autofluorescent and LysoTracker Red-stained acidified gut granules within intestinal cells, visualized in fluorescein isothiocyanate and rhodamine channels, respectively. Intestinal cells in young adult apt-6 (D–F), apt-7 (G–I), vps-16 (J–L), glo-1 (P–R), glo-2 (S–U), and glo-4 (Y–A′) animals contained few or no autofluorescent and acidified gut granules. vps-41 (M–O) animals contained a reduced number of gut granules that were localized in intestinal cells near the basal membrane. Reduced numbers of gut granules were present within the intestinal cells of glo-3 (V–X) animals. Posterior glo-3 intestinal cells (shown in V–X) contained on average 2 to 3 times the number of autofluorescent and acidic gut granules as anterior intestinal cells. In all panels the intestinal lumen (apical) is marked with a black arrow.
Figure 4.
Embryos require glo-1 for the assembly of acidified gut granules within the intestine. Intestinal cells in wild-type bean (A and B), 1.5-fold (C and D), and pretzel stage (E and F) embryos contained many acidic compartments stained by acridine orange. In wild type, the majority of gut granules were present within acidified compartments. (G–L) glo-1 intestinal cells at the same embryonic stages lacked birefringent gut granules and acidic structures stained by acridine orange. In all panels, the intestinal primordium is located between the black arrowheads. Acridine orange-stained apoptotic corpses anterior of the intestinal primordium in wild type (B and D) and glo-1 (H and J) embryos.
Figure 5.
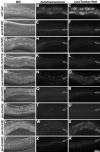
Elongation of wild-type and Glo mutant embryos. Images of wild-type (A–C) and glo-1 (D–F) embryos were captured at the times listed after first cell cleavage. glo-1 embryos elongated normally and reached the fourfold stage (E and G), however soon after they reduced to 3-fold in length (F and G) and displayed ripples in their epidermis/cuticle (F, black arrows). glo-2 (H) and glo-3 (I) embryos similarly elongated to fourfold before reducing to 3-fold length. (G–I) Glo embryos lacked birefringent gut granules before they occurred in the intestinal lumen (arrow denotes average time of occurrence). In G–I, the length of individual embryos and the presence/localization of birefringent material was monitored every 45 min starting at the 1.5-fold stage. Bars represent 95% confidence interval.
Figure 6.
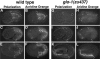
glo-1 is necessary for the formation of acidified and terminal endocytic organelles in larvae and adults. In wild type, gut granules were weakly autofluorescent in the rhodamine channel (B). After staining with dyes that label acidic (D) or terminal endocytic (F and H) compartments, gut granules in wild type accumulated the dyes and displayed much stronger fluorescence. In contrast, intestinal cells in glo-1 mutants did not stain with acidic (P) or terminal the endocytic (R) organelle marker TRITC-bovine serum albumin. In 25% (n = 20) of glo-1 animals, the endocytic marker FM4-64 accumulated in organelles localized near the apical surface of intestinal cells (T). In A–H and M–T, the intestinal lumen is marked with a black arrow. glo-1 mutants did not have defects in receptor mediated endocytosis (compare J and V) of YP170::GFP into oocytes (white arrowheads mark the most proximal oocytes). Fluid phase endocytosis of GFP localized within the body cavity by coelomocytes (white circle marks cell periphery) was not disrupted in glo-1 mutants (compare L and X).
Figure 7.
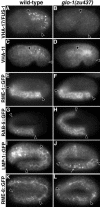
Analysis of endo-lysosomal organelles in glo-1 embryos. Antibodies that recognize the V-ATPase subunits VHA-17/FUS-1 (A and B) and VHA-11 (C and D) stained organelles in wild-type 1.5-fold (A) and bean-stage (C) embryos. These organelles were absent glo-1(–) embryos at similar stages (B and D). Anti-GFP antibodies used to stain embryos expressing RME-1 (E and F), RAB-5 (G and H) LMP-1 (I and J), and RME-8 (K and L) GFP protein fusions revealed a similar staining pattern in wild-type and glo-1(–) pretzel-stage embryos, with the exception that LMP-1::GFP structures were slightly enlarged in glo-1 embryos (white arrow in J). In all panels, intestinal cells are located between the black arrowheads.
Figure 8.
Predicted structure of the glo-1 gene and encoded protein. (A) The intron-exon structure of glo-1 and location of the glo-1 mutations are shown. (B) The predicted C. elegans GLO-1 protein sequence (AAY42967) is aligned to the C. briggsae GLO-1 (CBP14321 and CBP14320), D. melanogaster RabRP1 (AB035646), Dictyostelium RabE (AF116859), human Rab38 (NP071732), and human Rab32 (Q13637) proteins. Positions of GTPase (G1-G5), Rab family (F1–F5), and prenylation motifs are shown.
Figure 9.
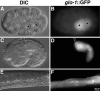
Expression of glo-1 in embryos and adults. (A–F) Wild-type strains carried an extrachromosomal transgene containing the glo-1 promoter driving the expression of gfp (glo-1::gfp). gfp expression in intestinal precursors at the E2 (A and B) and 1.5-fold (C and D) stages are shown. (A and B) Nuclei of intestinal precursors are marked with asterisks. (C and D) Black arrowheads denote the anterior of the intestinal primordium. (E and F) GFP was expressed within the adult intestine (black arrow marks lumen). Bar (E and F), 25 μm.
Figure 10.
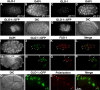
Subcellular localization of GLO-1. Wild-type (A and B) and glo-1(zu437) (C and D) E8 stage embryos stained with anti-GLO-1 antibodies. (E–H) glo-1(zu437) strains carrying an extrachromosomal transgene containing the glo-1 promoter driving the expression of GLO-1::GFP in intestinal precursor cells at the E4 (E and F) and E8 (G and H) stages. glo-1(zu437); GLO-1::GFP embryos at the E8 stage (I–L) and 1.5-fold stage (M-P) after staining with anti-GFP and anti-VHA-17/FUS-1 antibodies. GLO-1::GFP and FUS-1 colocalized to the same intestinal organelles (white arrows). (A–L) Black asterisks marks intestinal nuclei. (M–P) Black arrowheads mark the anterior of the intestine. (R–T) High magnification of the intestine of a 1.5-fold, glo-1(zu437); GLO-1::GFP embryo (boxed region in Q). Most gut granules, pseudocolored red in S and T, are present within vesicles containing GLO-1::GFP (R and T) (white arrows). Some gut granules are not present within GLO-1::GFP containing organelles (white arrowhead).
Similar articles
- Caenorhabditis elegans HOPS and CCZ-1 mediate trafficking to lysosome-related organelles independently of RAB-7 and SAND-1.
Delahaye JL, Foster OK, Vine A, Saxton DS, Curtin TP, Somhegyi H, Salesky R, Hermann GJ. Delahaye JL, et al. Mol Biol Cell. 2014 Apr;25(7):1073-96. doi: 10.1091/mbc.E13-09-0521. Epub 2014 Feb 5. Mol Biol Cell. 2014. PMID: 24501423 Free PMC article. - Function and regulation of the Caenorhabditis elegans Rab32 family member GLO-1 in lysosome-related organelle biogenesis.
Morris C, Foster OK, Handa S, Peloza K, Voss L, Somhegyi H, Jian Y, Vo MV, Harp M, Rambo FM, Yang C, Hermann GJ. Morris C, et al. PLoS Genet. 2018 Nov 12;14(11):e1007772. doi: 10.1371/journal.pgen.1007772. eCollection 2018 Nov. PLoS Genet. 2018. PMID: 30419011 Free PMC article. - An ABCG Transporter Functions in Rab Localization and Lysosome-Related Organelle Biogenesis in Caenorhabditis elegans.
Voss L, Foster OK, Harper L, Morris C, Lavoy S, Brandt JN, Peloza K, Handa S, Maxfield A, Harp M, King B, Eichten V, Rambo FM, Hermann GJ. Voss L, et al. Genetics. 2020 Feb;214(2):419-445. doi: 10.1534/genetics.119.302900. Epub 2019 Dec 17. Genetics. 2020. PMID: 31848222 Free PMC article. - Cell type-specific Rab32 and Rab38 cooperate with the ubiquitous lysosome biogenesis machinery to synthesize specialized lysosome-related organelles.
Bultema JJ, Di Pietro SM. Bultema JJ, et al. Small GTPases. 2013 Jan-Mar;4(1):16-21. doi: 10.4161/sgtp.22349. Epub 2012 Dec 17. Small GTPases. 2013. PMID: 23247405 Free PMC article. Review. - Who's in control? Principles of Rab GTPase activation in endolysosomal membrane trafficking and beyond.
Borchers AC, Langemeyer L, Ungermann C. Borchers AC, et al. J Cell Biol. 2021 Sep 6;220(9):e202105120. doi: 10.1083/jcb.202105120. Epub 2021 Aug 12. J Cell Biol. 2021. PMID: 34383013 Free PMC article. Review.
Cited by
- ICD-1/BTF3 antagonizes SKN-1-mediated endoderm specification in Caenorhabditis elegans.
Ewe CK, Torres Cleuren Y, Alok G, Rothman J. Ewe CK, et al. MicroPubl Biol. 2019 Oct 4;2019:10.17912/micropub.biology.000167. doi: 10.17912/micropub.biology.000167. MicroPubl Biol. 2019. PMID: 32550464 Free PMC article. No abstract available. - Analysis of centriole elimination during C. elegans oogenesis.
Mikeladze-Dvali T, von Tobel L, Strnad P, Knott G, Leonhardt H, Schermelleh L, Gönczy P. Mikeladze-Dvali T, et al. Development. 2012 May;139(9):1670-9. doi: 10.1242/dev.075440. Development. 2012. PMID: 22492357 Free PMC article. - RAB-10-GTPase-mediated regulation of endosomal phosphatidylinositol-4,5-bisphosphate.
Shi A, Liu O, Koenig S, Banerjee R, Chen CC, Eimer S, Grant BD. Shi A, et al. Proc Natl Acad Sci U S A. 2012 Aug 28;109(35):E2306-15. doi: 10.1073/pnas.1205278109. Epub 2012 Aug 6. Proc Natl Acad Sci U S A. 2012. PMID: 22869721 Free PMC article. - Lysosome-related organelles in intestinal cells are a zinc storage site in C. elegans.
Roh HC, Collier S, Guthrie J, Robertson JD, Kornfeld K. Roh HC, et al. Cell Metab. 2012 Jan 4;15(1):88-99. doi: 10.1016/j.cmet.2011.12.003. Cell Metab. 2012. PMID: 22225878 Free PMC article. - Evolutionary divergence of anaphase spindle mechanics in nematode embryos constrained by antagonistic pulling and viscous forces.
Khatri D, Brugière T, Athale CA, Delattre M. Khatri D, et al. Mol Biol Cell. 2022 May 15;33(6):ar61. doi: 10.1091/mbc.E21-10-0532. Epub 2022 Mar 2. Mol Biol Cell. 2022. PMID: 35235368 Free PMC article.
References
- Babu, P. (1974). Biochemical genetics of Caenorhabditis elegans. Mol. Gen. Genet. 135, 39–44.
- Blott, E. J., and Griffiths, G. M. (2002). Secretory lysosomes. Nat. Rev. Mol. Cell. Biol. 3, 122–131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous