Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing - PubMed (original) (raw)
Comparative Study
Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing
Keiichi Nagata et al. J Neurosci. 2005.
Abstract
Mechanosensory channels of sensory cells mediate the sensations of hearing, touch, and some forms of pain. The TRPA1 (a member of the TRP family of ion channel proteins) channel is activated by pain-producing chemicals, and its inhibition impairs hair cell mechanotransduction. As shown here and previously, TRPA1 is expressed by hair cells as well as by most nociceptors (small neurons of dorsal root, trigeminal, and nodose ganglia) and localizes to their sensory terminals (mechanosensory stereocilia and peripheral free nerves, respectively). Thus, TRPA1 channels are proposed to mediate transduction in both hair cells and nociceptors. Accordingly, we find that heterologously expressed TRPA1 display channel behaviors expected for both auditory and nociceptive transducers. First, TRPA1 and the hair cell transducer share a unique set of pore properties not described for any other channel (block by gadolinium, amiloride, gentamicin, and ruthenium red, a ranging conductance of approximately 100 pS that is reduced to 54% by calcium, permeating calcium-induced potentiation followed by closure, and reopening by depolarization), supporting a direct role of TRPA1 as a pore-forming subunit of the hair cell transducer. Second, TRPA1 channels inactivate in hyperpolarized cells but remain open in depolarized cells. This property provides a mechanism for the lack of desensitization, coincidence detection, and allodynia that characterize pain by allowing a sensory neuron to respond constantly to sustained stimulation that is suprathreshold (i.e., noxious) and yet permitting the same cell to ignore sustained stimulation that is subthreshold (i.e., innocuous). Our results support a TRPA1 role in both nociceptor and hair cell transduction.
Figures
Figure 1.
Mouse TRPA1 is expressed by most nociceptors and localized to peripheral sensory fibers. a-j, TRPA1 mRNA is detected by in situ hybridization in the majority of the nociceptors from dorsal root [n = 2904 cells from 29 sections of a postnatal day 37 (P37) CD1 mouse] (a-c), trigeminal (n = 988 cells from 19 sections of a P180 CBA/CAJ mouse) (d-f), and nodose ganglia (n = 504 cells from 20 sections of a young adult CD1 mouse) (g-i), but not in superior cervical ganglia (of a young adult CD1 mouse) (j), which lacks nociceptive neurons. k-m, With antibodies, TRPA1 protein (k) is also detected in the soma of small diameter, peripherin-positive nociceptive neurons from trigeminal ganglia (arrows) but not in the large diameter ones (arrowheads), as well as in the nociceptive sensory processes that innervate the bladder epithelium (l). m, The same antibodies detect an expected band of ∼128 kDa in Western blots from peripheral nerve lysate.
Figure 4.
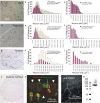
Agonists and antagonists of TRPA1 channels heterologously expressed in HEK 293 cells. a-c, Currents elicited by exposure of TRPA1 channels to AITC (a) (which appear complex, with a slow activation component followed by a faster one and then by inactivation) and icilin (b), but not by cold temperatures, are blocked by gentamicin, amiloride (AMIL), gadolinium, and ruthenium red (RR) (c, d). All blockers were tested in the absence of extracellular calcium to distinguish block from calcium-induced channel closure. d, Dose-response curves for gadolinium (n = 4), gentamicin (n = 4), and amiloride (number of cells given in parentheses next to data points). Error bars indicate SD.
Figure 2.
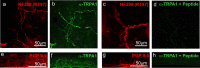
Mouse TRPA1 protein in nociceptive nerve fibers that innervate the trigone of the bladder (a-d) and the cornea (e-h). a-d, The nerves are labeled in red with anti-neurofilament [RT97 (for a, c) and PGP9.5 (for e, g)], and anti-TRPA1 immunoreactivity is indicated in green (b, f, d, h). b, f, TRPA1 immunoreactivity in the presence of GST protein. d, h, Elimination of the TRPA1 immunoreactivity in the presence of antigenic peptide (a fusion of GST to the N-terminal 60 residues of TRPA1).
Figure 3.
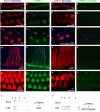
Mouse TRPA1 is expressed in the inner ear and localizes to the mechanosensory hair cell bundles. Anti-TRPA1 immunoreactivity is indicated in green; actin-rich hair cell bundles, labeled with phalloidin, are indicated in red; and kinocilia, labeled with anti-tubulin antibodies, are in blue. The two columns at the left show TRPA1 immunoreactivity in the presence of GST protein, whereas the two columns at the right show elimination of the TRPA1 immunoreactivity in the presence of antigenic peptide (a fusion of GST to the N-terminal 60 residues of TRPA1). TRPA1 immunoreactivity specifically labels the stereocilliary bundles of inner hair cells (a-d), outer hair cells (e-h), utricular hair cells (i-l), and hair cells of the crista ampullaris (m-p), as well as the apical side (the actin-rich cuticular plate) of outer hair cells (q-t). The label is not detected at the basal side of hair cells (not shown). u, v, Western blots reveal a single band detected by anti-TRPA1 on protein lysates from mouse saccule plus utricle (S+U) and organ of Corti (OC).
Figure 5.
Calcium-induced potentiation and inactivation of TRPA1 is voltage dependent, and the inactivation is reversed by sustained depolarization. a, b, In the absence (a) of external calcium, AITC elicits only slow activation (-80 mV holding potential), whereas in the presence (b) of external calcium, inactivation is complete at hyperpolarized (-80 mV) holding potentials but not at depolarized (-20 mV) potentials. c, Addition of calcium to the external medium of slowly activated TRPA1 channels elicits fast potentiation followed by inactivation (-80 mV holding potential). Notice that intracellular calcium is being chelated with 10 m
m
EGTA. d, The calcium-induced potentiation and subsequent inactivation of TRPA1 channels also occurs with high (2 and 3 m
m
) levels of internal calcium (introduced by diffusion from the pipette, under the whole-cell configuration, over a period of >5 min). e, Whole-cell recordings of TRPA1-expressing HEK cells exposed to several voltage steps reveal no voltage-dependent gating of TRPA1 channels in the range of -80 to +80 mV. f, However, calcium-induced inactivation, which is reduced at positive (+80 mV) membrane potentials (arrowhead) and maximal at negative (-80 mV) ones (arrow), is reversed by positive holding potentials (asterisk) after a delay of ∼30 s. The mechanism for this recovery from inactivation is presently unknown. No [Ca2+]o solutions contain 10 m
m
EGTA as chelator and have no CaCl2 added. For all panels, the trace shown is a representative of at least four separate cells.
Figure 6.
Single-channel properties of TRPA1. a, Recapitulation of TRPA1 slow activation, calcium-induced potentiation, and subsequent inactivation at the single-channel level in outside- out patches (at a holding potential of -80 mV). AITC, in the absence of calcium, activates a large conductance, flickery channel (II, activation) that, on exposure to calcium, transitions to a low conductance but high open-probability state (III, potentiation) followed by a low open-probability state (IV, inactivation). The bottom trace is a whole-cell record, obtained just before patch formation, indicating (in roman numerals) the times that correspond to the single-channel traces above. b, Transitions between low and high single-channel currents reveal a multitude of intermediate conductance levels for TRPA1 channels. Top trace, Cell-attached patch (with standard external solution in the pipette; amplitudes of 3.4, 4.1, 4.6, and 4.9 pA). Middle trace, Amplitude histograms of currents induced by application of AITC. Curves super-imposed on the histograms are five Gaussian fits to the data points. At least four conductance levels were discerned. Bottom trace, Outside- out patch at a holding potential of -80 mV (amplitudes ranging continuously from 6.4 to 3.3 pA). c, Current-voltage relationship of a representative TRPA1 channel, with a conductance of 98 pS at -60 mV, activated with 10 μ
m
AITC and recorded on a cell-attached patch with standard external solution in the pipette. For all panels, the trace shown is a representative of at least four separate membrane patches. Error bars in c represent confidence interval. C, Channels closed; O1, one channel open; O2, two channels open.
Similar articles
- A functional role for small-conductance calcium-activated potassium channels in sensory pathways including nociceptive processes.
Bahia PK, Suzuki R, Benton DC, Jowett AJ, Chen MX, Trezise DJ, Dickenson AH, Moss GW. Bahia PK, et al. J Neurosci. 2005 Apr 6;25(14):3489-98. doi: 10.1523/JNEUROSCI.0597-05.2005. J Neurosci. 2005. PMID: 15814779 Free PMC article. - The pore properties of human nociceptor channel TRPA1 evaluated in single channel recordings.
Bobkov YV, Corey EA, Ache BW. Bobkov YV, et al. Biochim Biophys Acta. 2011 Apr;1808(4):1120-8. doi: 10.1016/j.bbamem.2010.12.024. Epub 2010 Dec 29. Biochim Biophys Acta. 2011. PMID: 21195050 Free PMC article. - Transient receptor potential A1 mediates an osmotically activated ion channel.
Zhang XF, Chen J, Faltynek CR, Moreland RB, Neelands TR. Zhang XF, et al. Eur J Neurosci. 2008 Feb;27(3):605-11. doi: 10.1111/j.1460-9568.2008.06030.x. Eur J Neurosci. 2008. PMID: 18279313 - The transient receptor potential channel TRPA1: from gene to pathophysiology.
Nilius B, Appendino G, Owsianik G. Nilius B, et al. Pflugers Arch. 2012 Nov;464(5):425-58. doi: 10.1007/s00424-012-1158-z. Epub 2012 Sep 22. Pflugers Arch. 2012. PMID: 23001121 Review. - [Activation and regulation of nociceptive transient receptor potential (TRP) channels, TRPV1 and TRPA1].
Tominaga M. Tominaga M. Yakugaku Zasshi. 2010 Mar;130(3):289-94. doi: 10.1248/yakushi.130.289. Yakugaku Zasshi. 2010. PMID: 20190512 Review. Japanese.
Cited by
- Species-specific temperature sensitivity of TRPA1.
Laursen WJ, Anderson EO, Hoffstaetter LJ, Bagriantsev SN, Gracheva EO. Laursen WJ, et al. Temperature (Austin). 2015 Feb 11;2(2):214-26. doi: 10.1080/23328940.2014.1000702. eCollection 2015 Apr-Jun. Temperature (Austin). 2015. PMID: 27227025 Free PMC article. Review. - Bimodal effects of cinnamaldehyde and camphor on mouse TRPA1.
Alpizar YA, Gees M, Sanchez A, Apetrei A, Voets T, Nilius B, Talavera K. Alpizar YA, et al. Pflugers Arch. 2013 Jun;465(6):853-64. doi: 10.1007/s00424-012-1204-x. Epub 2012 Dec 28. Pflugers Arch. 2013. PMID: 23271453 - Peripherally increased artemin is a key regulator of TRPA1/V1 expression in primary afferent neurons.
Ikeda-Miyagawa Y, Kobayashi K, Yamanaka H, Okubo M, Wang S, Dai Y, Yagi H, Hirose M, Noguchi K. Ikeda-Miyagawa Y, et al. Mol Pain. 2015 Mar 8;11:8. doi: 10.1186/s12990-015-0004-7. Mol Pain. 2015. PMID: 25889103 Free PMC article. - TRPA1 channels in the vasculature.
Earley S. Earley S. Br J Pharmacol. 2012 Sep;167(1):13-22. doi: 10.1111/j.1476-5381.2012.02018.x. Br J Pharmacol. 2012. PMID: 22563804 Free PMC article. Review. - Warmth suppresses and desensitizes damage-sensing ion channel TRPA1.
Wang S, Lee J, Ro JY, Chung MK. Wang S, et al. Mol Pain. 2012 Mar 29;8:22. doi: 10.1186/1744-8069-8-22. Mol Pain. 2012. PMID: 22458587 Free PMC article.
References
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A (2004) Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41: 849-857. - PubMed
- Corey DP, García-Añoveros J, Holt JR, Kwan KY, Lin SY, Vollrath MA, Amalfitano A, Cheung EL, Derfler BH, Duggan A, Géléoc GS, Gray PA, Hoffman MP, Rehm HL, Tamasauskas D, Zhang DS (2004) TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 432: 723-730. - PubMed
- Denk W, Holt JR, Shepherd GM, Corey DP (1995) Calcium imaging of single stereocilia in hair cells: localization of transduction channels at both ends of tip links. Neuron 15: 1311-1321. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials