Unilateral storage of fear memories by the amygdala - PubMed (original) (raw)
Comparative Study
Unilateral storage of fear memories by the amygdala
Hugh T Blair et al. J Neurosci. 2005.
Erratum in
- J Neurosci. 2005 Oct 5;25(40):9317
Abstract
Pavlovian fear conditioning is an associative learning task in which subjects are trained to respond defensively to a neutral conditioned stimulus (CS) by pairing it with an aversive unconditioned stimulus (US). This type of learning depends critically on the amygdala, and evidence suggests that synaptic plasticity within the lateral nucleus of the amygdala (LA) may be responsible for storing memories of the CS-US association. In the present study, we trained rats to fear an auditory CS by pairing it with a shock US delivered to one eyelid. Conditioning was assessed by measuring freezing responses evoked by the CS during a subsequent test session. The amygdala was unilaterally inactivated during either the training or the testing session by intracranial infusions of muscimol into the LA. We found that both acquisition and expression of conditioned freezing to the CS depended on the amygdala contralateral but not ipsilateral from the eyelid where the shock US was delivered. To explain this surprising result, we propose that the shock US is relayed from the eyelid to the amygdala via lateralized nociceptive sensory pathways, which causes memories of the CS-US association to be stored by the amygdala contralateral but not ipsilateral from the shocked eyelid. Our results demonstrate that the fear-learning circuitry of the amygdala is functionally lateralized according to the anatomical source of predicted threats. In future studies, the cellular mechanisms of emotional memory storage might be pinpointed by identifying cellular processes that occur only in the amygdala contralateral but not ipsilateral from the US during lateralized fear conditioning.
Figures
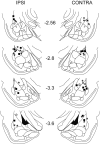
Figure 1.
Histological analysis of cannula placements. Locations of cannula tip placements where muscimol was delivered are shown (control placements where no drugs were delivered are omitted for clarity). All placement hemispheres are specified relative to the eyelid where the shock US was delivered during training. Squares indicate unilateral groups (IPSI/CONTRA), circles indicate the BI group, and asterisks indicate the PAIR group.
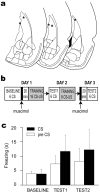
Figure 4.
Off-site control injections. a, Asterisks mark the site of muscimol injections into the caudate nucleus in the hemisphere contralateral from the shocked eyelid. b, Design of the experiment. c, Mean freezing scores for baseline and two test sessions. TEST1 was conducted drug-free 1 d after training with muscimol, and TEST2 was conducted with muscimol 1 d after drug-free training.
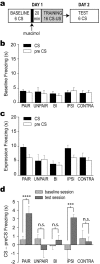
Figure 2.
Acquisition. a, Design of the acquisition experiment. b, Mean freezing scores (seconds of freezing) during the 20 s CS and pre-CS periods for each group of rats during the six trials of the baseline session. c, Mean freezing scores during the six trials of the expression test session. d, Mean freezing attributable specifically to the CS and not the training context (CS minus pre-CS freezing; see Results). Significance levels for Fisher's LSD tests: ****p < 0.0001; ***p < 0.001; n.s., not significant.
Figure 3.
Expression. a, Mean freezing score during the 20 s CS period on each day of the experiment. The subjects in the expression experiment were the rats from the paired group in the acquisition experiment, so days 1 and 2 are the acquisition experiment days. Days 3-8 are overtraining days (see Results), and days 9-15 are expression test days. Shading denotes days on which different amygdala hemispheres were inactivated before the test session. b, Pooled freezing scores for rats in both eyelid groups (left and right combined) during the pre-CS and CS periods for each of the four amygdala inactivation conditions of the expression test.
Similar articles
- The lateral amygdala processes the value of conditioned and unconditioned aversive stimuli.
Blair HT, Sotres-Bayon F, Moita MA, Ledoux JE. Blair HT, et al. Neuroscience. 2005;133(2):561-9. doi: 10.1016/j.neuroscience.2005.02.043. Neuroscience. 2005. PMID: 15878802 - Bilateral phosphorylation of ERK in the lateral and centrolateral amygdala during unilateral storage of fear memories.
Tarpley JW, Shlifer IG, Birnbaum MS, Halladay LR, Blair HT. Tarpley JW, et al. Neuroscience. 2009 Dec 15;164(3):908-17. doi: 10.1016/j.neuroscience.2009.08.071. Epub 2009 Sep 6. Neuroscience. 2009. PMID: 19735699 Free PMC article. - Role of the basolateral amygdala in the reinstatement and extinction of fear responses to a previously extinguished conditioned stimulus.
Laurent V, Westbrook RF. Laurent V, et al. Learn Mem. 2010 Feb 13;17(2):86-96. doi: 10.1101/lm.1655010. Print 2010 Feb. Learn Mem. 2010. PMID: 20154354 - The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction.
Walker DL, Davis M. Walker DL, et al. Pharmacol Biochem Behav. 2002 Mar;71(3):379-92. doi: 10.1016/s0091-3057(01)00698-0. Pharmacol Biochem Behav. 2002. PMID: 11830172 Review. - Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning.
Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Blair HT, et al. Learn Mem. 2001 Sep-Oct;8(5):229-42. doi: 10.1101/lm.30901. Learn Mem. 2001. PMID: 11584069 Review.
Cited by
- Reproductive experience and the response of female Sprague-Dawley rats to fear and stress.
Rima BN, Bardi M, Friedenberg JM, Christon LM, Karelina KE, Lambert KG, Kinsley CH. Rima BN, et al. Comp Med. 2009 Oct;59(5):437-43. Comp Med. 2009. PMID: 19887027 Free PMC article. - Acute cognitive impairment after lateral fluid percussion brain injury recovers by 1 month: evaluation by conditioned fear response.
Lifshitz J, Witgen BM, Grady MS. Lifshitz J, et al. Behav Brain Res. 2007 Feb 27;177(2):347-57. doi: 10.1016/j.bbr.2006.11.014. Epub 2006 Dec 13. Behav Brain Res. 2007. PMID: 17169443 Free PMC article. - Auditory trace fear conditioning requires perirhinal cortex.
Kholodar-Smith DB, Boguszewski P, Brown TH. Kholodar-Smith DB, et al. Neurobiol Learn Mem. 2008 Oct;90(3):537-43. doi: 10.1016/j.nlm.2008.06.006. Epub 2008 Aug 21. Neurobiol Learn Mem. 2008. PMID: 18678265 Free PMC article. - De novo inter-regional coactivations of preconfigured local ensembles support memory.
Miyawaki H, Mizuseki K. Miyawaki H, et al. Nat Commun. 2022 Mar 11;13(1):1272. doi: 10.1038/s41467-022-28929-x. Nat Commun. 2022. PMID: 35277492 Free PMC article. - Medial auditory thalamic nuclei are necessary for eyeblink conditioning.
Halverson HE, Freeman JH. Halverson HE, et al. Behav Neurosci. 2006 Aug;120(4):880-7. doi: 10.1037/0735-7044.120.4.880. Behav Neurosci. 2006. PMID: 16893294 Free PMC article.
References
- Baker KB, Kim JJ (2004) Amygdalar lateralization in fear conditioning: evidence for greater involvement of the right amygdala. Behav Neurosci 118: 15-23. - PubMed
- Bernard JF, Dallel R, Raboisson P, Villanueva L, Le Bars D (1995) Organization of the efferent projections from the spinal cervical enlargement to the parabrachial area and periaqueductal gray: a PHA-L study in the rat. J Comp Neurol 353: 480-505. - PubMed
- Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE (2001) Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learn Mem 8: 229-242. - PubMed
- Blanchard DC, Blanchard RJ (1969) Crouching as an index of fear. J Comp Physiol Psychol 67: 370-375. - PubMed
- Brunzell DH, Kim JJ (2001) Fear conditioning to tone, but not to context, is attenuated by lesions of the insular cortex and posterior extension of the intralaminar complex in rats. Behav Neurosci 115: 365-375. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical