Maspin regulates hypoxia-mediated stimulation of uPA/uPAR complex in invasive breast cancer cells - PubMed (original) (raw)
Comparative Study
Maspin regulates hypoxia-mediated stimulation of uPA/uPAR complex in invasive breast cancer cells
Sumaira Amir et al. Cancer Biol Ther. 2005 Apr.
Abstract
Maspin, a unique serine proteinase inhibitor (serpin), plays a key role in mammary gland development and is silenced during breast cancer progression. Maspin has been shown to inhibit tumor cell motility and invasion in cell culture, as well as growth and metastasis in animal models. In this study, we investigated the effect of maspin on the regulation of hypoxia-induced expression of urokinase-type plasminogen activator (uPA) and its receptor (uPAR), with respect to invasive potential in metastatic breast cells MDA-MB-231. We hypothesized that maspin can neutralize or mitigate hypoxia-induced expression of uPA/uPAR in metastatic breast cancer cells, resulting in suppression of their invasive potential. To test our hypothesis, we employed the highly invasive MDA-MB-231 breast cancer cells that are devoid of maspin, and transfected them with the maspin gene, and then determined the effect of hypoxia on uPA/uPAR expression. Normal mammary epithelial cells 1436N1 were used as a control. Our findings demonstrate that maspin downregulated the basal and hypoxia-induced uPA/uPAR expression and reduced the stimulatory effect of hypoxia on the in vitro invasive ability of MDA-MB-231-cells. In addition, maspin also inhibited the enzymatic activity of secreted and cell associated uPA in MDA-MB-231 cells. These results indicate that maspin inhibits hypoxia-induced invasion of metastatic breast cancer cells by blocking the uPA system, thus illuminating an important molecular pathway for therapeutic consideration.
Figures
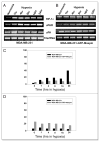
Figure 1
Effect of maspin on uPA and uPAR expression under hypoxia. MDA-MB-231 (A) and MDA-MB-231-GFP-Maspin (B) were cultured under hypoxia for (0–24 hrs). Total RNA was then isolated and analyzed by RT-PCR using uPAR, uPA and HIF-1α specific primers. 18s rRNA primers were used as control for equal loading. The signals for uPA and uPAR mRNA were quantified by densitometric analysis and represented as the ratio of uPA:18srRNA and uPAR:18s rRNA (C and D). Results shown in the lower panels are representatives of three independent experiments and were performed using Scion image analysis and Sigma plot.
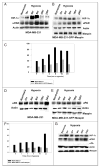
Figure 2
Maspin inhibits the uPAR and uPA expression in MDA-MB- 231-GFP-Maspin cells. Western blot analysis of uPAR and uPA protein in MDA-MB-231 (A) MDA-MB-231-GFP-maspin (B) and normal mammary epithelial cells 1436N1 (C) cultured for (0–24 hrs) under hypoxia. Equal amounts (25 μg) of cytosolic protein was resolved on a 10% sodium dodecyl sulfate-polyacrylamide gel, transblotted onto nitorcellulose membrane, and probed with anti-HIF-1α, uPAR and uPA monoclonal antibodies. Monoclonal antibody to actin was used as a control for equal loading. The data represents the densitometric analysis of results represented as the ratio of uPA: actin and uPAR:actin (C and F). Results shown in the lower panels were performed using Scion image and Sigma plot are representatives of three to five independent experiments.
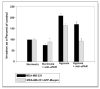
Figure 3
Maspin inhibits hypoxia-induced in vitro invasion in human breast cancer cells MDA-MB-231. Equal amounts (50,000) cells/wells were seeded into the upper wells of the MICS chamber containing Mito+ serum-free media, following a 24 hr incubation under either hypoxia or normoxia in the absence or presence of 20 μg/ml anti-uPAR neutralizing antibody. After 24 hrs the cells in the bottom chamber were harvested and counted. The percentage of invasion was determined by counting the number of cells that migrated through a collagen 1V/laminin/gelatin-coated polycarbonate fiter in 24 hr/total number of cells seeded X 100). The Data are expressed as a percentage of normoxic cells and are representatives of 3–4 independent experiments.
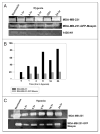
Figure 4
Maspin inhibits the activity of cell associated and secreted uPA in MDA-MB-231 and MDA-MB-231-GFP-Maspin cells. UPA activity of (A) cell-associated and secreted (C) in breast cancer cells MDA-MB-231, MDA-MB-231 GFP-maspin and normal mammary epithelial cells 1436N1 under hypoxia at indicated times as determined by zymography. Clear bands at 50–55 kDa represent enzymatic activity of uPA due to the activation of plasminogen. Levels of uPA activity were quantified by densitometry employing scion image analysis and sigma plot (B and D). Results shown in the lower panels are representatives of three independent experiments.
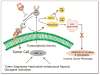
Figure 5
Hypothetical model for the regulation of uPA system by maspin under hypoxia in breast cancer cells. Exposure of cancer cells to hypoxic environment leads to increased secreation of prouPA which then binds to its cellular receptor uPAR. This interaction between uPA-uPAR provides inducible, transient and localized cell surface proteolytic activity, required for tissue invasion, by enhancing the conversion of plasminogen to plasmin. Plasmin can either directly degrade basement ECM or activate other zymogen proteases such as procollagenase, resulting in increase invasive potential of tumor cells. In this model maspin regulates the uPA system by blocking the interaction of uPA with uPAR by binding to uPA and inactivating it. The maspin-uPA complex together with uPAR gets internalized and degraded. Thus, inhibiting migration, invasion and ultimately metastasis by the highly invasive and metastatic breast cancer cells MDA-MB-231.
Comment in
- Hypoxia effects: implications for maspin regulation of the uPA/uPAR complex.
Schaefer JS, Zhang M. Schaefer JS, et al. Cancer Biol Ther. 2005 Sep;4(9):1033-5. doi: 10.4161/cbt.4.9.2057. Epub 2005 Sep 27. Cancer Biol Ther. 2005. PMID: 16123583
Similar articles
- Hypoxia effects: implications for maspin regulation of the uPA/uPAR complex.
Schaefer JS, Zhang M. Schaefer JS, et al. Cancer Biol Ther. 2005 Sep;4(9):1033-5. doi: 10.4161/cbt.4.9.2057. Epub 2005 Sep 27. Cancer Biol Ther. 2005. PMID: 16123583 - The surface of prostate carcinoma DU145 cells mediates the inhibition of urokinase-type plasminogen activator by maspin.
McGowen R, Biliran H Jr, Sager R, Sheng S. McGowen R, et al. Cancer Res. 2000 Sep 1;60(17):4771-8. Cancer Res. 2000. PMID: 10987285 - Maspin retards cell detachment via a novel interaction with the urokinase-type plasminogen activator/urokinase-type plasminogen activator receptor system.
Yin S, Lockett J, Meng Y, Biliran H Jr, Blouse GE, Li X, Reddy N, Zhao Z, Lin X, Anagli J, Cher ML, Sheng S. Yin S, et al. Cancer Res. 2006 Apr 15;66(8):4173-81. doi: 10.1158/0008-5472.CAN-05-3514. Cancer Res. 2006. PMID: 16618739 - Inhibition of the tumor-associated urokinase-type plasminogen activation system: effects of high-level synthesis of soluble urokinase receptor in ovarian and breast cancer cells in vitro and in vivo.
Magdolen V, Krüger A, Sato S, Nagel J, Sperl S, Reuning U, Rettenberger P, Magdolen U, Schmitt M. Magdolen V, et al. Recent Results Cancer Res. 2003;162:43-63. doi: 10.1007/978-3-642-59349-9_4. Recent Results Cancer Res. 2003. PMID: 12790320 Review. - Urokinase/urokinase receptor system: internalization/degradation of urokinase-serpin complexes: mechanism and regulation.
Conese M, Blasi F. Conese M, et al. Biol Chem Hoppe Seyler. 1995 Mar;376(3):143-55. Biol Chem Hoppe Seyler. 1995. PMID: 7612191 Review.
Cited by
- NAHA, a novel hydroxamic acid-derivative, inhibits growth and angiogenesis of breast cancer in vitro and in vivo.
Jiang J, Thyagarajan-Sahu A, Krchňák V, Jedinak A, Sandusky GE, Sliva D. Jiang J, et al. PLoS One. 2012;7(3):e34283. doi: 10.1371/journal.pone.0034283. Epub 2012 Mar 29. PLoS One. 2012. PMID: 22479587 Free PMC article. - Integrating the tumor-suppressive activity of Maspin with p53 in retuning the epithelial homeostasis: A working hypothesis and applicable prospects.
Tang S, Ling Z, Jiang J, Gu X, Leng Y, Wei C, Cheng H, Li X. Tang S, et al. Front Oncol. 2022 Nov 29;12:1037794. doi: 10.3389/fonc.2022.1037794. eCollection 2022. Front Oncol. 2022. PMID: 36523976 Free PMC article. Review. - Snail transcription factor negatively regulates maspin tumor suppressor in human prostate cancer cells.
Neal CL, Henderson V, Smith BN, McKeithen D, Graham T, Vo BT, Odero-Marah VA. Neal CL, et al. BMC Cancer. 2012 Aug 2;12:336. doi: 10.1186/1471-2407-12-336. BMC Cancer. 2012. PMID: 22857708 Free PMC article. - p53 dominant-negative mutant R273H promotes invasion and migration of human endometrial cancer HHUA cells.
Dong P, Tada M, Hamada J, Nakamura A, Moriuchi T, Sakuragi N. Dong P, et al. Clin Exp Metastasis. 2007;24(6):471-83. doi: 10.1007/s10585-007-9084-8. Epub 2007 Jul 18. Clin Exp Metastasis. 2007. PMID: 17636407 - Maspin increases extracellular plasminogen activator activity associated with corneal fibroblasts and myofibroblasts.
Warejcka DJ, Narayan M, Twining SS. Warejcka DJ, et al. Exp Eye Res. 2011 Nov;93(5):618-27. doi: 10.1016/j.exer.2011.07.008. Epub 2011 Jul 27. Exp Eye Res. 2011. PMID: 21810423 Free PMC article.
References
- Movsas B, Chapman JD, Greenberg RE, Hanlon AL, Horwitz EM, Pinover WH, Stobbe C, Hanks GE. Increasing levels of hypoxia in prostate carcinoma correlate significantly with increasing clinical stage and patient age. Cancer Res. 2000;89:2018–24. - PubMed
- Brown JM. The Hypoxic Cell. A target for selective cancer therapy. Cancer Res. 1999;59:5863–70. - PubMed
- Teicher BA. Hypoxia and drug resistance. Cancer Metastasis Rev. 1998;13:139–68. - PubMed
- Cuvier C, Jang A, Hill RP. Exposure to hypoxia, glucose starvation and acidosis: Effect on invasive capacity of murine tumor cells and correlation with cathepsin (L + B) secretion. Clin Exp Metastasis. 1997;15:19–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous