Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance - PubMed (original) (raw)
Clinical Trial
Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance
Steven Haffner et al. Diabetes. 2005 May.
Abstract
Increases in subclinical inflammation (C-reactive protein [CRP]) and impaired coagulation have been associated with increased obesity and insulin resistance. Only a few small studies have examined the effect of lifestyle changes, such as weight loss, increased physical activity, and insulin-sensitizing intervention on inflammation and coagulation. The Diabetes Prevention Program (DPP) clinical trial studied the effect of an intensive lifestyle intervention or metformin on progression to diabetes relative to placebo in 3,234 adults with impaired glucose tolerance. The effects of these interventions on CRP and fibrinogen at 12 months are examined in this report. Metformin reduced CRP in women compared with the placebo group. In men, the median changes in CRP from baseline to 1 year were -33% in the lifestyle group, -7% in the metformin group, and +5% in the placebo group. In women, the changes in CRP from baseline to follow-up were -29% in the lifestyle group, -14% in the metformin group, and 0% in the placebo group. In the lifestyle group weight loss rather than increased physical activity seems to account for most of the changes in CRP. Only modest reductions (although significant) were seen in fibrinogen levels in the lifestyle group relative to the metformin and placebo group. Lifestyle intervention reduced levels of nontraditional cardiovascular risk factors relative to both placebo and to a lesser degree to metformin.
Figures
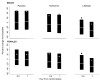
FIG. 1
Median percentage of change in CRP from baseline at month 6 and year 1 by sex. The data are presented as mean (gray dot), median (white line), and 25th (top of the gray box) and 75th percentiles (bottom of the gray box). At 6 months and at year 1 after randomization, the Wilcoxon’s test yielded P < 0.001 for intensive lifestyle vs. metformin and intensive lifestyle vs. placebo in both men and women. After 6 months and 1 year of intervention, the P value from the Wilcoxon’s test between metformin vs. placebo was <0.001 for women. Among men, the Wilcoxon’s test for comparing metformin vs. placebo yielded P = 0.01 at 6 months and 0.006 at year 1.
Similar articles
- Impact of Lifestyle and Metformin Interventions on the Risk of Progression to Diabetes and Regression to Normal Glucose Regulation in Overweight or Obese People With Impaired Glucose Regulation.
Herman WH, Pan Q, Edelstein SL, Mather KJ, Perreault L, Barrett-Connor E, Dabelea DM, Horton E, Kahn SE, Knowler WC, Lorenzo C, Pi-Sunyer X, Venditti E, Ye W; Diabetes Prevention Program Research Group. Herman WH, et al. Diabetes Care. 2017 Dec;40(12):1668-1677. doi: 10.2337/dc17-1116. Epub 2017 Oct 11. Diabetes Care. 2017. PMID: 29021207 Free PMC article. Clinical Trial. - Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the diabetes prevention program.
Ratner R, Goldberg R, Haffner S, Marcovina S, Orchard T, Fowler S, Temprosa M; Diabetes Prevention Program Research Group. Ratner R, et al. Diabetes Care. 2005 Apr;28(4):888-94. doi: 10.2337/diacare.28.4.888. Diabetes Care. 2005. PMID: 15793191 Free PMC article. Clinical Trial. - Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions.
Ratner RE, Christophi CA, Metzger BE, Dabelea D, Bennett PH, Pi-Sunyer X, Fowler S, Kahn SE; Diabetes Prevention Program Research Group. Ratner RE, et al. J Clin Endocrinol Metab. 2008 Dec;93(12):4774-9. doi: 10.1210/jc.2008-0772. Epub 2008 Sep 30. J Clin Endocrinol Metab. 2008. PMID: 18826999 Free PMC article. Clinical Trial. - An update on the Diabetes Prevention Program.
Ratner RE; Diabetes Prevention Program Research. Ratner RE, et al. Endocr Pract. 2006 Jan-Feb;12 Suppl 1(Suppl 1):20-4. doi: 10.4158/EP.12.S1.20. Endocr Pract. 2006. PMID: 16627375 Free PMC article. Review.
Cited by
- Cardiometabolic profiles and proteomics associated with obesity phenotypes in a longitudinal cohort of young adults.
Liao J, Goodrich JA, Chen W, Qiu C, Chen JC, Costello E, Alderete TL, Chatzi L, Gilliland F, Chen Z. Liao J, et al. Sci Rep. 2024 Mar 28;14(1):7384. doi: 10.1038/s41598-024-57751-2. Sci Rep. 2024. PMID: 38548792 Free PMC article. - Association between systemic immune-inflammation index and insulin resistance and mortality.
Deng X, Liu D, Li M, He J, Fu Y. Deng X, et al. Sci Rep. 2024 Jan 23;14(1):2013. doi: 10.1038/s41598-024-51878-y. Sci Rep. 2024. PMID: 38263234 Free PMC article. - Metformin: The Winding Path from Understanding Its Molecular Mechanisms to Proving Therapeutic Benefits in Neurodegenerative Disorders.
Isop LM, Neculau AE, Necula RD, Kakucs C, Moga MA, Dima L. Isop LM, et al. Pharmaceuticals (Basel). 2023 Dec 11;16(12):1714. doi: 10.3390/ph16121714. Pharmaceuticals (Basel). 2023. PMID: 38139841 Free PMC article. Review. - Combined effect of adiposity and elevated inflammation on incident type 2 diabetes: a prospective cohort study.
Wu D, Lan Y, Chen S, Ding X, Chen G, Wu C, Balmer L, Xu W, Wu S, Wang W. Wu D, et al. Cardiovasc Diabetol. 2023 Dec 20;22(1):351. doi: 10.1186/s12933-023-02067-0. Cardiovasc Diabetol. 2023. PMID: 38124083 Free PMC article. - Physiopathological mechanisms related to inflammation in obesity and type 2 diabetes mellitus.
Lempesis IG, Georgakopoulou VE. Lempesis IG, et al. World J Exp Med. 2023 Jun 20;13(3):7-16. doi: 10.5493/wjem.v13.i3.7. eCollection 2023 Jun 20. World J Exp Med. 2023. PMID: 37396883 Free PMC article. Review.
References
- UKPDS Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. - PubMed
- Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Cardiovascular risk factors in confirmed prediabetic individuals: does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA. 1990;263:2893–2898. - PubMed
- Fagot-Campagna A, Narayan KM, Hanson RL, Imperatore G, Howard BV, Nelson RG, Pettitt DJ, Knowler WC. Plasma lipoproteins and incidence of non-insulin-dependent diabetes mellitus in Pima Indians: protective effect of HDL cholesterol in women. Atherosclerosis. 1997;128:113–119. - PubMed
- Festa A, D′ Agostino R, Jr, Tracy RP, Haffner SM. Elevated levels of acute phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the Insulin Resistance Atherosclerosis Study. Diabetes. 2002;51:1131–1137. - PubMed
- Hu FB, Stampfer MJ, Haffner SM, Solomon CG, Willett WC, Manson JE. Elevated risk of cardiovascular disease prior to clinical diagnosis of type 2 diabetes. Diabetes Care. 2002;25:1129–1134. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous