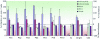Human serum facilitates hepatitis C virus infection, and neutralizing responses inversely correlate with viral replication kinetics at the acute phase of hepatitis C virus infection - PubMed (original) (raw)
Comparative Study
Human serum facilitates hepatitis C virus infection, and neutralizing responses inversely correlate with viral replication kinetics at the acute phase of hepatitis C virus infection
Dimitri Lavillette et al. J Virol. 2005 May.
Abstract
The factors leading to spontaneous clearance of hepatitis C virus (HCV) or to viral persistence are elusive. Understanding virus-host interactions that enable acute HCV clearance is key to the development of more effective therapeutic and prophylactic strategies. Here, using a sensitive neutralization assay based on infectious HCV pseudoparticles (HCVpp), we have studied the kinetics of humoral responses in a cohort of acute-phase patients infected during a single nosocomial outbreak in a hemodialysis center. The 17 patients were monitored for the spontaneous outcome of HCV infection for 6 months before a treatment decision was made. Blood samples were taken frequently (15 +/- 4 per patient). Phylogenetic analysis of the predominant virus(es) revealed infection by only one of two genotype 1b strains. While all patients seroconverted, their sera induced two opposing effects in HCVpp infection assays: inhibition and facilitation. Furthermore, the ability of sera to facilitate or inhibit infection correlated with the presence of either infecting HCV strain and divided the patients into two groups. In group 1, the progressive emergence of a relatively strong neutralizing response correlated with a fluctuating decrease in high initial viremia, leading to control of viral replication. Patients in group 2 failed to reduce viremia within the acute phase, and no neutralizing responses were detected despite seroconversion. Strikingly, sera of group 2, as well as naive sera, facilitated infection by HCVpp displaying HCV glycoproteins from different genotypes and strains, including those retrieved from patients. These results provide new insights into the mechanisms of viral persistence and immune control of viremia.
Figures
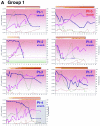
FIG. 1.
ALT levels, seroconversion patterns, and kinetics of HCV RNA and of neutralizing and facilitating response levels in our cohort of acutely HCV-infected hemodialysis patients. Individual ALT kinetics (green curves; arbitrary units) and HCV RNA kinetics (blue curves) were measured approximately on a weekly basis following inclusion of the patients in the cohort. The individual patients' kinetics are shown. The coded names for patients infected with virus strain A and strain B (Fig. 2) are highlighted in blue and red, respectively. Patients from group 1 (Fig. 1A) exhibited significant HCV RNA fluctuations that ultimately led to control of viral replication. Patients from group 2 (Fig. 2B) exhibited sustained high replication levels throughout the entire study period. Seroconversion patterns were characterized with the INNO-LIA HCV IV line immunoassay that detects antibodies against HCV structural and nonstructural proteins. The appearance of and increase in antibody titers are shown as colored lines (color code: yellow, low antibody titer, to brown, high antibody titer) at the top of each patient's diagram for the eight testedHCV antigens (from top to bottom: core 1, core 2, E1, E2, NS3, NS4A, NS4B, and NS5). The effect of each serum sample on the infectivity of HCV genotype 1b pseudoparticles (HCVpp, strain CG1b) was analyzed by incubating identical ratios of viral particles (104 i.u.) and sera (1/50 dilution) for 30 min at room temperature before infection of Huh-7 target cells. The results (pink curves) are expressed as the mean percentages of inhibition of the average infectious titers relative to incubation with medium devoid of human serum. The results were derived from at least three independent experiments using different virion production batches, and the standard deviations (not shown for sake of clarity) did not exceed 30% of the mean values. Since some sera facilitated infection, the resulting infectivity was higher than that of HCVpp incubated with human serum-free medium and consequently raised negative values when expressed as percentages of inhibition. The baseline that separated neutralization (red areas; positive values) and facilitation (green areas; negative values) is shown as dotted lines. The specificity of either phenomenon was addressed by using control pseudoparticles generated with the irrelevant target RD114 glycoprotein from a feline endogenous virus (RD114pp), for which no antibodies are detected in human sera (3). As expected, nonspecific inhibition or facilitation of the control RD114pp (brown curves) was never detected over a value of ±20%. Patients Pt-4, Pt-9, and Pt-11 were treated with pegylated alpha interferon a, 135 μg weekly, during the follow-up period for various reasons explained in the text (gray areas), resulting in significant HCV RNA load decreases.
FIG. 1.
ALT levels, seroconversion patterns, and kinetics of HCV RNA and of neutralizing and facilitating response levels in our cohort of acutely HCV-infected hemodialysis patients. Individual ALT kinetics (green curves; arbitrary units) and HCV RNA kinetics (blue curves) were measured approximately on a weekly basis following inclusion of the patients in the cohort. The individual patients' kinetics are shown. The coded names for patients infected with virus strain A and strain B (Fig. 2) are highlighted in blue and red, respectively. Patients from group 1 (Fig. 1A) exhibited significant HCV RNA fluctuations that ultimately led to control of viral replication. Patients from group 2 (Fig. 2B) exhibited sustained high replication levels throughout the entire study period. Seroconversion patterns were characterized with the INNO-LIA HCV IV line immunoassay that detects antibodies against HCV structural and nonstructural proteins. The appearance of and increase in antibody titers are shown as colored lines (color code: yellow, low antibody titer, to brown, high antibody titer) at the top of each patient's diagram for the eight testedHCV antigens (from top to bottom: core 1, core 2, E1, E2, NS3, NS4A, NS4B, and NS5). The effect of each serum sample on the infectivity of HCV genotype 1b pseudoparticles (HCVpp, strain CG1b) was analyzed by incubating identical ratios of viral particles (104 i.u.) and sera (1/50 dilution) for 30 min at room temperature before infection of Huh-7 target cells. The results (pink curves) are expressed as the mean percentages of inhibition of the average infectious titers relative to incubation with medium devoid of human serum. The results were derived from at least three independent experiments using different virion production batches, and the standard deviations (not shown for sake of clarity) did not exceed 30% of the mean values. Since some sera facilitated infection, the resulting infectivity was higher than that of HCVpp incubated with human serum-free medium and consequently raised negative values when expressed as percentages of inhibition. The baseline that separated neutralization (red areas; positive values) and facilitation (green areas; negative values) is shown as dotted lines. The specificity of either phenomenon was addressed by using control pseudoparticles generated with the irrelevant target RD114 glycoprotein from a feline endogenous virus (RD114pp), for which no antibodies are detected in human sera (3). As expected, nonspecific inhibition or facilitation of the control RD114pp (brown curves) was never detected over a value of ±20%. Patients Pt-4, Pt-9, and Pt-11 were treated with pegylated alpha interferon a, 135 μg weekly, during the follow-up period for various reasons explained in the text (gray areas), resulting in significant HCV RNA load decreases.
FIG. 1.
ALT levels, seroconversion patterns, and kinetics of HCV RNA and of neutralizing and facilitating response levels in our cohort of acutely HCV-infected hemodialysis patients. Individual ALT kinetics (green curves; arbitrary units) and HCV RNA kinetics (blue curves) were measured approximately on a weekly basis following inclusion of the patients in the cohort. The individual patients' kinetics are shown. The coded names for patients infected with virus strain A and strain B (Fig. 2) are highlighted in blue and red, respectively. Patients from group 1 (Fig. 1A) exhibited significant HCV RNA fluctuations that ultimately led to control of viral replication. Patients from group 2 (Fig. 2B) exhibited sustained high replication levels throughout the entire study period. Seroconversion patterns were characterized with the INNO-LIA HCV IV line immunoassay that detects antibodies against HCV structural and nonstructural proteins. The appearance of and increase in antibody titers are shown as colored lines (color code: yellow, low antibody titer, to brown, high antibody titer) at the top of each patient's diagram for the eight testedHCV antigens (from top to bottom: core 1, core 2, E1, E2, NS3, NS4A, NS4B, and NS5). The effect of each serum sample on the infectivity of HCV genotype 1b pseudoparticles (HCVpp, strain CG1b) was analyzed by incubating identical ratios of viral particles (104 i.u.) and sera (1/50 dilution) for 30 min at room temperature before infection of Huh-7 target cells. The results (pink curves) are expressed as the mean percentages of inhibition of the average infectious titers relative to incubation with medium devoid of human serum. The results were derived from at least three independent experiments using different virion production batches, and the standard deviations (not shown for sake of clarity) did not exceed 30% of the mean values. Since some sera facilitated infection, the resulting infectivity was higher than that of HCVpp incubated with human serum-free medium and consequently raised negative values when expressed as percentages of inhibition. The baseline that separated neutralization (red areas; positive values) and facilitation (green areas; negative values) is shown as dotted lines. The specificity of either phenomenon was addressed by using control pseudoparticles generated with the irrelevant target RD114 glycoprotein from a feline endogenous virus (RD114pp), for which no antibodies are detected in human sera (3). As expected, nonspecific inhibition or facilitation of the control RD114pp (brown curves) was never detected over a value of ±20%. Patients Pt-4, Pt-9, and Pt-11 were treated with pegylated alpha interferon a, 135 μg weekly, during the follow-up period for various reasons explained in the text (gray areas), resulting in significant HCV RNA load decreases.
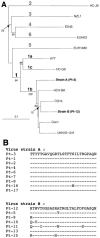
FIG. 2.
HCV strain genotyping. (A) Phylogenetic analyses of full-length E1-E2 amino acid sequences from HCV strain A, infecting patient Pt-3, and HCV strain B, infecting patient Pt-12. The phylogenetic tree was constructed by the neighbor-joining method with a sequence matrix determined by a Kimura approach (PRODIST and NEIGHBOR software). The tree is artificially rooted using an HC-J6 isolate (prototype genotype 1b) as the outgroup. HCV types and subtypes are indicated above their respective branches. Only bootstrap values of >50% are indicated below the branches for 1,000 replicates.For each of the reference sequences, the accession number is given in Materials and Methods. (B) Alignment of amino acid residues of HVR1 of the HCV E2 glycoprotein. For each patient, HVR1 sequences were obtained by direct sequencing and corresponded to the major variant present in the first available serum. Amino acid sequences are shown using the one-letter code. Dashes represent residues identical to the top sequence.
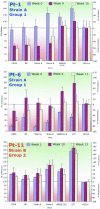
FIG.3.
Detection of HCV cross-neutralization. Pseudoparticles generated with E1-E2 glycoproteins of genotype 1b (strains CG1b, BK, and UKN1B.12.6, as well as strains A and B, respectively, derived from patients Pt-3 and Pt-11) or 1a (strain H77) or with RD114 glycoproteins were incubated with selected sera from patients Pt-1 (weeks 0, 9, and 15), Pt-6 (weeks 0, 4, and 13), and Pt-11 (weeks 1, 10, and 13). The results are expressed on the left y axis as percentages of the average infectious titers plus standard deviations relative to titers determined in the absence of human serum. Therefore, values of <100% represent inhibition of infectivity (red areas) (indicated on the right y axis as percent neutralization), whereas values over this baseline (dotted line) show facilitation of HCVpp infection by human sera (green areas) (indicated on the right y axis as percent facilitation). The results were derived from at least three independent experiments using different virion production batches.
FIG. 4.
Facilitation of HCV infection by sera from non-HCV-infected donors and patients from group 2. Pseudoparticles generated with E1-E2 glycoproteins of genotype 1a (strain H) or 1b (strain CG1b), as well as with RD114, VSV-G, or hemagglutinin glycoproteins (FPV-HA), were incubated with sera derived from healthy donors (PS1 to PS4) or from selected sera of patients who did not display detectable neutralizing antibodies (Pt-8 at week 12, Pt-9 at week 10, and Pt-10 at week 15). The C23 neutralizing mouse monoclonal antibody and Vu, a serum from a chronic HCV carrier, were used as positive controls, as previously described. Except for pseudoparticles generated with VSV-G, which are inhibited by human complement (50), these experiments were carried out with sera containing complement activity. Heat treatment of these sera did not eliminate the facilitation of infection (data not shown). Depletion of IgG from the sera resulted in loss of neutralization activity (Vu IgG−). The results are expressed on the left y axis as percentages of the average infectious titers plus standard deviations relative to titers determined in the absence of human serum. Therefore, values of <100% represent inhibition of infectivity (red areas) (indicated on the right y axis as percent neutralization), whereas values over this baseline (dotted line) show facilitation of HCVpp infection by human sera (green areas) (indicated on the right y axis as percent facilitation). The results were derived from at least three independent experiments using different virion production batches.
Similar articles
- Effects of interferon treatment on the antiviral T-cell response in hepatitis C virus genotype 1b- and genotype 2c-infected patients.
Missale G, Cariani E, Lamonaca V, Ravaggi A, Rossini A, Bertoni R, Houghton M, Matsuura Y, Miyamura T, Fiaccadori F, Ferrari C. Missale G, et al. Hepatology. 1997 Sep;26(3):792-7. doi: 10.1053/jhep.1997.v26.pm0009303515. Hepatology. 1997. PMID: 9303515 - Modulation of epitope-specific anti-hepatitis C virus E2 (anti-HCV/E2) antibodies by anti-viral treatment.
Mancini N, Carletti S, Perotti M, Romanò L, Craxì RD, Craxì A, Zanetti AR, Clementi M, Burioni R. Mancini N, et al. J Med Virol. 2006 Oct;78(10):1304-11. doi: 10.1002/jmv.20704. J Med Virol. 2006. PMID: 16927283 Clinical Trial. - Review: Occult hepatitis C virus infection: still remains a controversy.
Vidimliski PD, Nikolov I, Geshkovska NM, Dimovski A, Rostaing L, Sikole A. Vidimliski PD, et al. J Med Virol. 2014 Sep;86(9):1491-8. doi: 10.1002/jmv.23979. Epub 2014 Jun 4. J Med Virol. 2014. PMID: 24895180 Review.
Cited by
- Hepatitis C virus vaccine design: focus on the humoral immune response.
Sepulveda-Crespo D, Resino S, Martinez I. Sepulveda-Crespo D, et al. J Biomed Sci. 2020 Jul 6;27(1):78. doi: 10.1186/s12929-020-00669-4. J Biomed Sci. 2020. PMID: 32631318 Free PMC article. Review. - Hepatitis C virus: virology, diagnosis and management of antiviral therapy.
Chevaliez S, Pawlotsky JM. Chevaliez S, et al. World J Gastroenterol. 2007 May 7;13(17):2461-6. doi: 10.3748/wjg.v13.i17.2461. World J Gastroenterol. 2007. PMID: 17552030 Free PMC article. Review. - Effect of hepatitis C virus core protein on the molecular profiling of human B lymphocytes.
Wu CG, Budhu A, Chen S, Zhou X, Popescu NC, Valerie K, Wang XW. Wu CG, et al. Mol Med. 2006 Jan-Mar;12(1-3):47-53. doi: 10.2119/2006-00020.Wu. Mol Med. 2006. PMID: 16838065 Free PMC article. - Adaptive immunity to the hepatitis C virus.
Walker CM. Walker CM. Adv Virus Res. 2010;78:43-86. doi: 10.1016/B978-0-12-385032-4.00002-1. Adv Virus Res. 2010. PMID: 21040831 Free PMC article. Review. - Clearance of hepatitis C infection is associated with the early appearance of broad neutralizing antibody responses.
Osburn WO, Snider AE, Wells BL, Latanich R, Bailey JR, Thomas DL, Cox AL, Ray SC. Osburn WO, et al. Hepatology. 2014 Jun;59(6):2140-51. doi: 10.1002/hep.27013. Epub 2014 Apr 29. Hepatology. 2014. PMID: 24425349 Free PMC article.
References
- Adams, G., S. Kuntz, G. Rabalais, D. Bratcher, C. H. Tamburro, and G. J. Kotwal. 1997. Natural recovery from acute hepatitis C virus infection by agammaglobulinemic twin children. Pediatr. Infect. Dis. J. 16:533-534. - PubMed
- Bartosch, B., J. Bukh, J. C. Meunier, C. Granier, R. E. Engle, W. C. Blackwelder, S. U. Emerson, F. L. Cosset, and R. H. Purcell. 2003. In vitro assay for neutralizing antibody to hepatitis C virus: evidence for broadly conserved neutralization epitopes. Proc. Natl. Acad. Sci. USA 100:14199-14204. - PMC - PubMed
- Bjoro, K., S. S. Froland, Z. Yun, H. H. Samdal, and T. Haaland. 1994. Hepatitis C infection in patients with primary hypogammaglobulinemia after treatment with infected immune globulin. N. Engl. J. Med. 331:1607-1611. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases