Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer's disease - PubMed (original) (raw)
Comparative Study
Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer's disease
Paul A Adlard et al. J Neurosci. 2005.
Abstract
Alzheimer's disease (AD) is a progressive neurodegenerative disorder for which there are few therapeutics that affect the underlying disease mechanism. Recent epidemiological studies, however, suggest that lifestyle changes may slow the onset/progression of AD. Here we have used TgCRND8 mice to examine directly the interaction between exercise and the AD cascade. Five months of voluntary exercise resulted in a decrease in extracellular amyloid-beta (Abeta) plaques in the frontal cortex (38%; p = 0.018), the cortex at the level of the hippocampus (53%; p = 0.0003), and the hippocampus (40%; p = 0.06). This was associated with decreased cortical Abeta1-40 (35%; p = 0.005) and Abeta1-42 (22%; p = 0.04) (ELISA). The mechanism appears to be mediated by a change in the processing of the amyloid precursor protein (APP) after short-term exercise, because 1 month of activity decreased the proteolytic fragments of APP [for alpha-C-terminal fragment (alpha-CTF), 54% and p = 0.04; for beta-CTF, 35% and p = 0.03]. This effect was independent of mRNA/protein changes in neprilysin and insulin-degrading enzyme and, instead, may involve neuronal metabolism changes that are known to affect APP processing and to be regulated by exercise. Long-term exercise also enhanced the rate of learning of TgCRND8 animals in the Morris water maze, with significant (p < 0.02) reductions in escape latencies over the first 3 (of 6) trial days. In support of existing epidemiological studies, this investigation demonstrates that exercise is a simple behavioral intervention sufficient to inhibit the normal progression of AD-like neuropathology in the TgCRND8 mouse model.
Figures
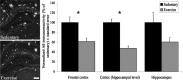
Figure 1.
The effect of exercise on Aβ load in three different brain regions of TgCRND8 mice. Exercising animals show a significant (*) reduction in Aβ immunoreactivity in the frontal cortex (p = 0.018) and cortex at the level of the hippocampus (p = 0.0003). There is also a decrease in Aβ in the hippocampus of exercising animals (p = 0.06). The photomicrograph shows a representative section from both groups. Scale bar, 200 μm. Error bars indicate ± SE.
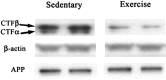
Figure 2.
Representative Western blot data from four animals showing no difference in APP or β-actin but showing significant (p < 0.04) reductions in both the α-CTF and β-CTF in the exercising animals compared with the sedentary animals.
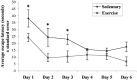
Figure 3.
The effect of exercise on Morris water maze performance. Average escape latencies for sedentary and exercising TgCRND8 animals in the Morris water maze are shown. Exercised animals show improved performance across the time course, with significant differences present on the first 3 d of trials, compared with the sedentary animals (*p < 0.02). Error bars indicate ± SE.
Similar articles
- A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model.
Lim GP, Calon F, Morihara T, Yang F, Teter B, Ubeda O, Salem N Jr, Frautschy SA, Cole GM. Lim GP, et al. J Neurosci. 2005 Mar 23;25(12):3032-40. doi: 10.1523/JNEUROSCI.4225-04.2005. J Neurosci. 2005. PMID: 15788759 Free PMC article. - Cerebrolysin decreases amyloid-beta production by regulating amyloid protein precursor maturation in a transgenic model of Alzheimer's disease.
Rockenstein E, Torrance M, Mante M, Adame A, Paulino A, Rose JB, Crews L, Moessler H, Masliah E. Rockenstein E, et al. J Neurosci Res. 2006 May 15;83(7):1252-61. doi: 10.1002/jnr.20818. J Neurosci Res. 2006. PMID: 16511867 - Self-assembling nanofibers alter the processing of amyloid precursor protein in a transgenic mouse model of Alzheimer's disease.
Yang H, Yang H, Xie Z, Wang P, Bi J. Yang H, et al. Neurol Res. 2015 Jan;37(1):84-91. doi: 10.1179/1743132814Y.0000000417. Epub 2014 Jul 9. Neurol Res. 2015. PMID: 25005263 - Amyloid precursor protein (APP) and the biology of proteolytic processing: relevance to Alzheimer's disease.
Ling Y, Morgan K, Kalsheker N. Ling Y, et al. Int J Biochem Cell Biol. 2003 Nov;35(11):1505-35. doi: 10.1016/s1357-2725(03)00133-x. Int J Biochem Cell Biol. 2003. PMID: 12824062 Review. - Genetic programming by the proteolytic fragments of the amyloid precursor protein: somewhere between confusion and clarity.
Stein TD, Johnson JA. Stein TD, et al. Rev Neurosci. 2003;14(4):317-41. doi: 10.1515/revneuro.2003.14.4.317. Rev Neurosci. 2003. PMID: 14640319 Review.
Cited by
- PET amyloid-beta imaging in preclinical Alzheimer's disease.
Vlassenko AG, Benzinger TL, Morris JC. Vlassenko AG, et al. Biochim Biophys Acta. 2012 Mar;1822(3):370-9. doi: 10.1016/j.bbadis.2011.11.005. Epub 2011 Nov 12. Biochim Biophys Acta. 2012. PMID: 22108203 Free PMC article. Review. - The effect of cognitive-motor dual-task training on cognitive function and plasma amyloid β peptide 42/40 ratio in healthy elderly persons: a randomized controlled trial.
Yokoyama H, Okazaki K, Imai D, Yamashina Y, Takeda R, Naghavi N, Ota A, Hirasawa Y, Miyagawa T. Yokoyama H, et al. BMC Geriatr. 2015 May 28;15:60. doi: 10.1186/s12877-015-0058-4. BMC Geriatr. 2015. PMID: 26018225 Free PMC article. Clinical Trial. - Associations of healthy lifestyles with cerebrospinal fluid biomarkers of Alzheimer's disease pathology in cognitively intact older adults: the CABLE study.
Hou XH, Xu W, Bi YL, Shen XN, Ma YH, Dong Q, Tan L, Yu JT. Hou XH, et al. Alzheimers Res Ther. 2021 Apr 19;13(1):81. doi: 10.1186/s13195-021-00822-7. Alzheimers Res Ther. 2021. PMID: 33875016 Free PMC article. - Exercise preconditioning improves traumatic brain injury outcomes.
Taylor JM, Montgomery MH, Gregory EJ, Berman NE. Taylor JM, et al. Brain Res. 2015 Oct 5;1622:414-29. doi: 10.1016/j.brainres.2015.07.009. Epub 2015 Jul 9. Brain Res. 2015. PMID: 26165153 Free PMC article. - Sex Differences in the White Matter and Myelinated Fibers of APP/PS1 Mice and the Effects of Running Exercise on the Sex Differences of AD Mice.
Zhou CN, Chao FL, Zhang Y, Jiang L, Zhang L, Luo YM, Xiao Q, Chen LM, Tang Y. Zhou CN, et al. Front Aging Neurosci. 2018 Aug 17;10:243. doi: 10.3389/fnagi.2018.00243. eCollection 2018. Front Aging Neurosci. 2018. PMID: 30174598 Free PMC article.
References
- Adlard PA, Perreau VM, Engesser-Cesar C, Cotman CW (2004) The time-course of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neurosci Lett 363: 43-48. - PubMed
- Caccamo A, Oddo S, Billings LM, Blurton-Jones M, Fisher A, LaFerla FM (2004) M1 muscarinic agonist treatment rescues the behavioral deficit present in the 3XTg-AD mice. Soc Neurosci Abstr 30: 675.18.
- Chishti MA, Yang DS, Janus C, Phinney AL, Home P, Pearson J, Strome R, Zuker N, Loukides J, French J, Turner S, Lozza G, Grilli M, Kunicki S, Morissette C, Paquette J, Gervais F, Bergeron C, Fraser PE, Carlson GA, et al. (2001) Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem 276: 21562-21570. - PubMed
- Cotman CW, Berchtold NC (2002) Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci 25: 295-301. - PubMed
- Cummings BJ, Cotman CW (1995) Image analysis of beta-amyloid load in Alzheimer's disease and relation to dementia severity. Lancet 346: 1524-1528. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases