Microglia overexpressing the macrophage colony-stimulating factor receptor are neuroprotective in a microglial-hippocampal organotypic coculture system - PubMed (original) (raw)
Comparative Study
Microglia overexpressing the macrophage colony-stimulating factor receptor are neuroprotective in a microglial-hippocampal organotypic coculture system
Olivera M Mitrasinovic et al. J Neurosci. 2005.
Abstract
Microglia with increased expression of the macrophage colony-stimulating factor receptor (M-CSFR; c-fms) are found surrounding plaques in Alzheimer's disease (AD) and in mouse models for AD and after ischemic or traumatic brain injury. Increased expression of M-CSFR causes microglia to adopt an activated state that results in proliferation, release of cytokines, and enhanced phagocytosis. To determine whether M-CSFR-induced microglial activation affects neuronal survival, we assembled a coculture system consisting of BV-2 microglia transfected to overexpress the M-CSFR and hippocampal organotypic slices treated with NMDA. Twenty-four hours after assembly of the coculture, microglia overexpressing M-CSFR proliferated at a higher rate than nontransfected control cells and exhibited enhanced migration toward NMDA-injured hippocampal cultures. Surprisingly, coculture with c-fms-transfected microglia resulted in a dramatic reduction in NMDA-induced neurotoxicity. Similar results were observed when cocultures were treated with the teratogen cyclophosphamide. Biolistic overexpression of M-CSFR on microglia endogenous to the organotypic culture also rescued neurons from excitotoxicity. Furthermore, c-fms-transfected microglia increased neuronal expression of macrophage colony-stimulating factor (M-CSF), the M-CSFR, and neurotrophin receptors in the NMDA-treated slices, as determined with laser capture microdissection. In the coculture system, direct contact between the exogenous microglia and the slice was necessary for neuroprotection. Finally, blocking expression of the M-CSF ligand by exogenous c-fms-transfected microglia with a hammerhead ribozyme compromised their neuroprotective properties. These results demonstrate a protective role for microglia overexpressing M-CSFR in our coculture system and suggest under certain circumstances, activated microglia can help rather than harm neurons subjected to excitotoxic and teratogen-induced injury.
Figures
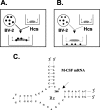
Figure 1.
A, Schematic diagram of one-level coculture system. Hcs, Hippocampal cultures. B, Two-level coculture system. C, RNA secondary sequence of the anti-M-CSF hammerhead ribozyme (Rz). Cleavage occurs after nucleotide 266 (M-CSF mRNA sequence NM 007778), as indicated by the arrow.
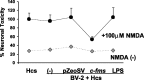
Figure 2.
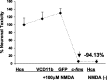
Neuroprotective effects of BV-2 microglia overexpressing M-CSFR in the microglial organotypic coculture system after NMDA treatment. The black data points represent NMDA-treated cocultures, whereas the gray data points represent cocultures not treated with NMDA. The _y_-axis represents PI staining calculated as a percentage of the staining seen hippocampal cultures (Hcs) alone (no microglia) treated with NMDA. This value is compared with a coculture system containing nontransfected microglia treated with transfection medium alone (-), microglia transfected with the control plasmid (pZeoSV), the c-fms plasmid (c-fms), or pretreated with LPS (LPS). Only c-_fms_-transfected exogenous microglia were neuroprotective. Error bars represent SD.
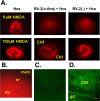
Figure 3.
PI labeling of neurotoxicity in the microglial-hippocampal coculture system. A, Left column, Hippocampal cultures (Hcs) treated with 5 or 100 μ
m
NMDA, no BV-2 cells. Middle column, Hippocampal cultures treated with 5 or 100 μ
m
NMDA and cocultured with BV-2 microglia transfected with c-fms construct. Right column, Hippocampal cultures treated with 5 or 100 μ
m
NMDA and cocultured with BV-2 microglia treated with transfection medium alone (-). B, High-power view of the edge of microglial-hippocampal organotypic coculture treated with NMDA and containing wild-type BV-2 cells. The strong PI staining is in the pyramidal layer (py) of CA1. The stratum oriens (or) shows no staining, and the Millicell membrane (mem) adjacent to the slice, although containing numerous BV-2 cells, shows no PI signal. C, Confocal image of section from a microglial-organotypic coculture containing BV-2 cells transfected to overexpress the M-CSFR and treated with NMDA and stained with FluoroJade. There was no FluoroJade signal visible using acquisition settings identical to those in D. In C, the overall brightness was increased after image acquisition to make the CA3 and granule cell layer landmarks faintly visible. D, Confocal images of section from sister coculture stained with FluoroJade. The coculture contained wild-type BV-2 cells transfected to overexpress the M-CSFR and was treated with 100 μ
m
NMDA. Note the strong neuronal staining in the CA3 and granule cell layers (gcl). Scale bar, 100 μm.
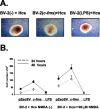
Figure 5.
Microglial proliferation induced by c-fms transfection and NMDA treatment in organotypic cocultures. A, Phase-contrast images of microglial-organotypic cocultures treated with NMDA. The middle panel shows strong proliferation of BV-2 cells transfected with c-fms [BV-2 (c-fms) + Hcs] compared with nontransfected cells [BV-2 (-) + Hcs] and BV-2 cells pretreated with LPS [BV-2 (LPS) + Hcs]. B, Quantitative analysis of total BV-2 cell numbers per well in cocultures containing BV-2 cells transfected with the control plasmid (pZeoSV), the c-fms plasmid, or pretreated with LPS, with (right) and without (left) the addition of NMDA, after 24 and 48 h. Hcs, Hippocampal cultures. Error bars represent SD.
Figure 4.
Biolistic expression of the M-CSFR on microglia in organotypic hippocampal cultures protects neurons from NMDA. The _y_-axis shows PI signal expressed as a percentage of nontransfected hippocampal cultures (Hcs). Other conditions included biolistic transfection with the control vector (VCD11b), with the plasmid containing an EGFP cDNA (GFP), with the plasmid resulting in overexpression of M-CSFR on microglia in the slice culture (c-fms), or nontransfected slices not treated with NMDA [Hcs NMDA (-)]. Error bars represent SD.
Figure 6.
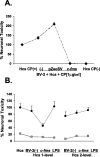
A, Neuroprotection by c-_fms_-transfected exogenous microglia in the microglial-organotypic coculture system after treatment with the teratogen CP. Data points represent PI signal for CP-treated hippocampal cultures alone [Hcs CP (+)] and cocultures with nontransfected BV-2 cells (-), control vector transfected (pZeoSV), and c-fms transfected (c-fms). Also shown are results for untreated hippocampal cocultures containing nontransfected BV-2 cells (Hcs CP-). All data are expressed relative to PI signal in Hcs alone treated with CP. B, Loss of neuroprotection by BV-2 microglia overexpressing the M-CSFR in a two-level coculture system. Black data points represent PI signal in NMDA-treated cultures, whereas gray data points show signal for cocultures not treated with NMDA. All data are expressed as a percentage of PI signal in hippocampal cultures (Hcs) alone treated with NMDA. BV-2 (-), Nontransfected cells; c-fms, c-_fms_-transfected cells; LPS, LPS-pretreated BV-2 cells. Error bars represent SD.
Figure 7.
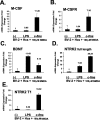
The effects of exogenous microglia on gene expression in neurons in microglia-organotypic cocultures treated with NMDA. A-E show results from neurons obtained with laser capture microdissection of cocultures. Data are presented for neuronal expression of M-CSF, M-CSFR, BDNF, NTRK2 full-length isoform, and NTRK2 T1 isoform. For each target, real-time RT-PCR results are shown for LPS-pretreated and c-_fms_-transfected BV-2 cells relative to expression levels in cocultures containing nontransfected BV-2 cells (-). Hcs, Hippocampal cultures. Error bars represent SD.
Figure 8.
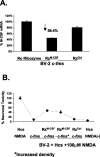
A, Decreased M-CSF expression by BV-2 microglia in monotypic cultures cotransfected with the c-fms plasmid and with the anti-M-CSF ribozyme (RzM-CSF). Data are also presented for a control ribozyme (RzCtrl). B, Attenuation of neuroprotective effect of BV-2 c-fms transfection in microglial-organotypic cocultures by cotransfection with the anti-M-CSF ribozyme. The _y_-axis represents PI signal relative to that in hippocampal cultures (Hcs) treated with NMDA alone (first data point). Also shown are results for coculture containing c-_fms_-transfected cells (second data point), c-_fms_-transfected cells treated with the anti-M-CSF ribozyme (third data point), c-_fms_-transfected BV-2 cells treated with the anti-M-CSF ribozyme and seeded at increased density (fourth data point), c-_fms_-transfected cells treated with the control ribozyme (fifth data point), and Hcs without cocultured BV-2 cells and without NMDA (sixth data point). The increased density data point represents cocultures containing numbers of ribozyme-treated cells equal to those typically found in a c-_fms_-transfected coculture not treated with the ribozyme. Error bars represent SD.
Similar articles
- Overexpression of macrophage colony-stimulating factor receptor on microglial cells induces an inflammatory response.
Mitrasinovic OM, Perez GV, Zhao F, Lee YL, Poon C, Murphy GM Jr. Mitrasinovic OM, et al. J Biol Chem. 2001 Aug 10;276(32):30142-9. doi: 10.1074/jbc.M104265200. Epub 2001 May 31. J Biol Chem. 2001. PMID: 11387343 - Biolistic expression of the macrophage colony stimulating factor receptor in organotypic cultures induces an inflammatory response.
Mitrasinovic OM, Robinson CC, Tenen DG, Lee YL, Poon C, Murphy GM Jr. Mitrasinovic OM, et al. J Neurosci Res. 2004 Aug 1;77(3):420-9. doi: 10.1002/jnr.20168. J Neurosci Res. 2004. PMID: 15248298 - Accelerated phagocytosis of amyloid-beta by mouse and human microglia overexpressing the macrophage colony-stimulating factor receptor.
Mitrasinovic OM, Murphy GM Jr. Mitrasinovic OM, et al. J Biol Chem. 2002 Aug 16;277(33):29889-96. doi: 10.1074/jbc.M200868200. Epub 2002 May 24. J Biol Chem. 2002. PMID: 12032144 - Expression of macrophage colony-stimulating factor and its receptor in microglia activation is linked to teratogen-induced neuronal damage.
Hao AJ, Dheen ST, Ling EA. Hao AJ, et al. Neuroscience. 2002;112(4):889-900. doi: 10.1016/s0306-4522(02)00144-6. Neuroscience. 2002. PMID: 12088748 - Neuronal Death: Now You See It, Now You Don't.
Balena T, Staley K. Balena T, et al. Neuroscientist. 2024 Sep 24:10738584241282632. doi: 10.1177/10738584241282632. Online ahead of print. Neuroscientist. 2024. PMID: 39316584 Review.
Cited by
- Effects of blast overpressure on neurons and glial cells in rat organotypic hippocampal slice cultures.
Miller AP, Shah AS, Aperi BV, Budde MD, Pintar FA, Tarima S, Kurpad SN, Stemper BD, Glavaski-Joksimovic A. Miller AP, et al. Front Neurol. 2015 Feb 12;6:20. doi: 10.3389/fneur.2015.00020. eCollection 2015. Front Neurol. 2015. PMID: 25729377 Free PMC article. - Uncovering molecular biomarkers that correlate cognitive decline with the changes of hippocampus' gene expression profiles in Alzheimer's disease.
Gómez Ravetti M, Rosso OA, Berretta R, Moscato P. Gómez Ravetti M, et al. PLoS One. 2010 Apr 13;5(4):e10153. doi: 10.1371/journal.pone.0010153. PLoS One. 2010. PMID: 20405009 Free PMC article. - Oral route lipopolysaccharide as a potential dementia preventive agent inducing neuroprotective microglia.
Mizobuchi H. Mizobuchi H. Front Immunol. 2023 Mar 9;14:1110583. doi: 10.3389/fimmu.2023.1110583. eCollection 2023. Front Immunol. 2023. PMID: 36969154 Free PMC article. Review. - Interleukin-34 restores blood-brain barrier integrity by upregulating tight junction proteins in endothelial cells.
Jin S, Sonobe Y, Kawanokuchi J, Horiuchi H, Cheng Y, Wang Y, Mizuno T, Takeuchi H, Suzumura A. Jin S, et al. PLoS One. 2014 Dec 23;9(12):e115981. doi: 10.1371/journal.pone.0115981. eCollection 2014. PLoS One. 2014. PMID: 25535736 Free PMC article. - Receptors on Microglia.
Augusto-Oliveira M, Tremblay MÈ, Verkhratsky A. Augusto-Oliveira M, et al. Adv Neurobiol. 2024;37:83-121. doi: 10.1007/978-3-031-55529-9_6. Adv Neurobiol. 2024. PMID: 39207688 Review.
References
- Aschner M, Allen JW, Kimelberg HK, LoPachin RM, Streit WJ (1999) Glial cells in neurotoxicity development. Annu Rev Pharmacol Toxicol 39: 151-173. - PubMed
- Bamberger ME, Landreth GE (2002) Inflammation, apoptosis, and Alzheimer's disease. Neuroscientist 8: 276-283. - PubMed
- Berezovskaya O, Maysinger D, Fedoroff S (1995) The hematopoietic cytokine, colony-stimulating factor 1, is also a growth factor in the CNS: congenital absence of CSF-1 in mice results in abnormal microglial response and increased neuron vulnerability to injury. Int J Dev Neurosci 13: 285-299. - PubMed
- Berezovskaya O, Maysinger D, Fedoroff S (1996) Colony stimulating factor-1 potentiates neuronal survival in cerebral cortex ischemic lesion. Acta Neuropathol (Berl) 92: 479-486. - PubMed
- Bruccoleri A, Harry GJ (2000) Chemical-induced hippocampal neurodegeneration and elevations in TNFalpha, TNFbeta, IL-1alpha, IP-10, and MCP-1 mRNA in osteopetrotic (op/op) mice. J Neurosci Res 62: 146-155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous