Differential requirements for the activation domain and FOG-interaction surface of GATA-1 in megakaryocyte gene expression and development - PubMed (original) (raw)
Differential requirements for the activation domain and FOG-interaction surface of GATA-1 in megakaryocyte gene expression and development
Andrew G Muntean et al. Blood. 2005.
Abstract
GATA1 is mutated in patients with 2 different disorders. First, individuals with a GATA1 mutation that blocks the interaction between GATA-1 and its cofactor Friend of GATA-1 (FOG-1) suffer from dyserythropoietic anemia and thrombocytopenia. Second, children with Down syndrome who develop acute megakaryoblastic leukemia harbor mutations in GATA1 that lead to the exclusive expression of a shorter isoform named GATA-1s. To determine the effect of these patient-specific mutations on GATA-1 function, we first compared the gene expression profile between wild-type and GATA-1-deficient megakaryocytes. Next, we introduced either GATA-1s or a FOG-binding mutant (V205G) into GATA-1-deficient megakaryocytes and assessed the effect on differentiation and gene expression. Whereas GATA-1-deficient megakaryocytes failed to undergo terminal differentiation and proliferated excessively in vitro, GATA-1s-expressing cells displayed proplatelet formation and other features of terminal maturation, but continued to proliferate aberrantly. In contrast, megakaryocytes that expressed V205G GATA-1 exhibited reduced proliferation, but failed to undergo maturation. Examination of the expression of megakaryocyte-specific genes in the various rescued cells correlated with the observed phenotypic differences. These studies show that GATA-1 is required for both normal regulation of proliferation and terminal maturation of megakaryocytes, and further, that these functions can be uncoupled by mutations in GATA1.
Figures
Figure 1.
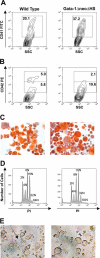
Characterization of ΔneoΔHS Gata1 knockdown megakaryocytes. (A-B) Flow cytometric analysis of megakaryocytes cultured from wild-type or GATA-1 knockdown fetal liver cells stained with the anti-CD41 (A) or CD42 (B) antibody. (C) Following in vitro expansion, wild-type and GATA-1–deficient cells were enriched on a BSA gradient and AChE staining was performed to determine the purity of enriched cells. Images were obtained using a Leica DM4000B light microscope equipped with a 20×/0.04 objective lens (Cambridge, United Kingdom). Original magnification, ×200. Images were captured using a mounted Leica DFC320 camera and Leica Firecam version 1.4 software. (D) Ploidy analysis of BSA gradient-purified megakaryocytes was performed by flow cytometry of cells stained with PI. (E) Light microscopy of differentiated cells in culture. The arrow points to proplatelet forms. Images were obtained using a Zeiss Axiovert S100 inverted microscope equipped with a 20×/0.04 objective lens (Thornwood, NY). Original magnification, ×200. Images were captured with a Nikon Coolpix 990 digital camera (Tokyo, Japan).
Figure 2.
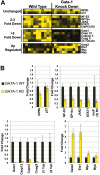
Requirements for GATA-1 in megakaryocyte gene expression. (A) Affymetrix probe set hybridization results for 16 representative genes that were differentially affected by the absence of GATA-1. Highest levels of expression are depicted as yellow squares; the lowest levels or no expression are represented by black squares. (B) Quantitative real-time RT-PCR validation of 16 genes selected from the array data. Data were divided into the following groups: genes that were not significantly affected (P > .05, upper left), ones that were down-regulated 2- to 3-fold (P ranged from < .002 to .02, upper right), those that were reduced more than 5-fold (P < .001, lower left), and genes that were induced in the absence of GATA-1 (GATA-2 and Ets-1, P < .001; myc P < .05; myb P < .5; lower right). Fold changes of gene expression in the GATA-1 knockdown cells (yellow bars) are shown relative to the levels detected in wild-type cells (black bars: wild-type expression was set to 1). Means ± SD for 3 experiments are shown.
Figure 3.
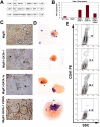
GATA-1s rescues proplatelet formation, but not the hyperproliferative phenotype. (A) Constructs used in this study. Wild-type or mutant GATA-1 cDNAs were introduced into the MIGR1 vector backbone, which includes an IRES-GFP cassette. (B) GATA-1 expression in the rescued population was determined by qRT-PCR. There was no statistically significant difference between full-length GATA-1 and GATA-1s expression in the reconstitutions, but there was a significant difference between GATA-1 and V205G expression (P < .02). Means ± SD for 3 experiments are shown. (C) Light microscopy for the generation of proplatelet forms by the rescued cells. Notable features include the presence of proplatelets in the GATA-1s rescued cells and the presence of very large megakaryocytes in the population expressing V205G. Original magnification × 320. Images were obtained as described in the legend of Figure 1E. (D) AChE-stained cytospins of an aliquot of the 3-day cultures. Note the presence of a very large AChE-stained megakaryocyte in the population expressing V205G. Original magnification × 200. Images were obtained as described in the legend of Figure 1C. (E) Cell surface expression of CD41 on megakaryocytes after 3 days of in vitro differentiation, assayed by flow cytometry.
Figure 4.
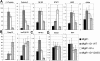
Megakaryocyte genes fall into several classes based on their requirement for different GATA-1 functional domains. Quantitative RT-PCR analysis of expression of 10 genes in the different rescued populations. These genes fell into 4 classes: (A) genes rescued by wild-type GATA-1 and GATA-1s but not by V205G, (B) genes rescued by GATA-1 and both GATA-1s and V205G, (C) genes rescued by GATA-1 but not GATA-1s or V205G, and (D) genes not significantly affected by wild-type or either of the GATA-1 mutants. Expression in knockdown cells infected with the MIGR1 retrovirus (black bars, far left) was set to 1, whereas the fold changes observed in the wild-type (dark gray bars), GATA-1s (white bars) and V205G (light gray bars, far right) reconstitutions are shown. Means ± SD for 3 experiments are shown.
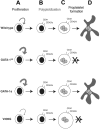
Figure 5.
Model of the differential requirements for GATA-1 in megakaryocyte gene expression and development. Committed megakaryocyte progenitors (A) normally display limited proliferation. However, in the absence of GATA-1, or in the presence of GATA-1s, these progenitors hyperproliferate and produce a greater number of CD41+ megakaryocytes, which also express low levels of CD42 (B). In contrast, when the ability of GATA-1 to interact with FOG-1 is disrupted, progenitors expand to a lesser degree. Megakaryocytes then undergo repeated rounds of DNA synthesis without cell division (polyploidization) to generate a polyploid cell (C). Mutagenesis of GATA-1 did not affect the endomitosis of megakaryocytes. However, alterations in GATA-1 led to differential effects on terminal maturation. In the absence of GATA-1, or in the presence of the V205G mutant, terminal maturation was blocked. When GATA-1s was expressed, however, megakaryocytes produced proplatelet forms (D) and were similar to wild-type cells in morphology and gene expression.
Similar articles
- Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - A mutation in the translation initiation codon of Gata-1 disrupts megakaryocyte maturation and causes thrombocytopenia.
Majewski IJ, Metcalf D, Mielke LA, Krebs DL, Ellis S, Carpinelli MR, Mifsud S, Di Rago L, Corbin J, Nicola NA, Hilton DJ, Alexander WS. Majewski IJ, et al. Proc Natl Acad Sci U S A. 2006 Sep 19;103(38):14146-51. doi: 10.1073/pnas.0606439103. Epub 2006 Sep 11. Proc Natl Acad Sci U S A. 2006. PMID: 16966598 Free PMC article. - Early block to erythromegakaryocytic development conferred by loss of transcription factor GATA-1.
Stachura DL, Chou ST, Weiss MJ. Stachura DL, et al. Blood. 2006 Jan 1;107(1):87-97. doi: 10.1182/blood-2005-07-2740. Epub 2005 Sep 6. Blood. 2006. PMID: 16144799 Free PMC article. - Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.
Lee CC, Chen CW, Yen HK, Lin YP, Lai CY, Wang JL, Groot OQ, Janssen SJ, Schwab JH, Hsu FM, Lin WH. Lee CC, et al. Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924 - Pharmacological treatments in panic disorder in adults: a network meta-analysis.
Guaiana G, Meader N, Barbui C, Davies SJ, Furukawa TA, Imai H, Dias S, Caldwell DM, Koesters M, Tajika A, Bighelli I, Pompoli A, Cipriani A, Dawson S, Robertson L. Guaiana G, et al. Cochrane Database Syst Rev. 2023 Nov 28;11(11):CD012729. doi: 10.1002/14651858.CD012729.pub3. Cochrane Database Syst Rev. 2023. PMID: 38014714 Free PMC article. Review.
Cited by
- GATA1 in Normal and Pathologic Megakaryopoiesis and Platelet Development.
Takasaki K, Chou ST. Takasaki K, et al. Adv Exp Med Biol. 2024;1459:261-287. doi: 10.1007/978-3-031-62731-6_12. Adv Exp Med Biol. 2024. PMID: 39017848 Review. - Enhancement of PRMT6 binding to a novel germline GATA1 mutation associated with congenital anemia.
Lu Y, Zhu Q, Wang Y, Luo M, Huang J, Liang Q, Huang L, Ouyang J, Li C, Tang N, Li Y, Kang T, Song Y, Xu X, Ye L, Zheng G, Chen C, Zhu C. Lu Y, et al. Haematologica. 2024 Sep 1;109(9):2955-2968. doi: 10.3324/haematol.2023.284183. Haematologica. 2024. PMID: 38385251 Free PMC article. - Prothymosin α accelerates dengue virus-induced thrombocytopenia.
Yang ML, Lin CL, Chen YC, Lu IA, Su BH, Chen YH, Liu KT, Wu CL, Shiau AL. Yang ML, et al. iScience. 2023 Dec 2;27(1):108422. doi: 10.1016/j.isci.2023.108422. eCollection 2024 Jan 19. iScience. 2023. PMID: 38213625 Free PMC article. - The novel GATA1-interacting protein HES6 is an essential transcriptional cofactor for human erythropoiesis.
Wang Z, Wang P, Zhang J, Gong H, Zhang X, Song J, Nie L, Peng Y, Li Y, Peng H, Cui Y, Li H, Hu B, Mi J, Liang L, Liu H, Zhang J, Ye M, Yazdanbakhsh K, Mohandas N, An X, Han X, Liu J. Wang Z, et al. Nucleic Acids Res. 2023 Jun 9;51(10):4774-4790. doi: 10.1093/nar/gkad167. Nucleic Acids Res. 2023. PMID: 36929421 Free PMC article. - Deciphering transcriptome alterations in bone marrow hematopoiesis at single-cell resolution in immune thrombocytopenia.
Liu Y, Zuo X, Chen P, Hu X, Sheng Z, Liu A, Liu Q, Leng S, Zhang X, Li X, Wang L, Feng Q, Li C, Hou M, Chu C, Ma S, Wang S, Peng J. Liu Y, et al. Signal Transduct Target Ther. 2022 Oct 7;7(1):347. doi: 10.1038/s41392-022-01167-9. Signal Transduct Target Ther. 2022. PMID: 36202780 Free PMC article.
References
- Crispino JD. GATA1 in normal and malignant hematopoiesis. Semin Cell Dev Biol. 2005;16: 137-147. - PubMed
- Vyas P, Ault K, Jackson CW, Orkin SH, Shivdasani RA. Consequences of GATA-1 deficiency in megakaryocytes and platelets. Blood. 1999;93: 2867-2875. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases