Inflammation, stress, and diabetes - PubMed (original) (raw)
Review
Inflammation, stress, and diabetes
Kathryn E Wellen et al. J Clin Invest. 2005 May.
Abstract
Over the last decade, an abundance of evidence has emerged demonstrating a close link between metabolism and immunity. It is now clear that obesity is associated with a state of chronic low-level inflammation. In this article, we discuss the molecular and cellular underpinnings of obesity-induced inflammation and the signaling pathways at the intersection of metabolism and inflammation that contribute to diabetes. We also consider mechanisms through which the inflammatory response may be initiated and discuss the reasons for the inflammatory response in obesity. We put forth for consideration some hypotheses regarding important unanswered questions in the field and suggest a model for the integration of inflammatory and metabolic pathways in metabolic disease.
Figures
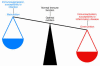
Figure 1
Metabolism and immunity are closely linked. Both overnutrition and undernutrition have implications for immune function. Starvation and malnutrition can suppress immune function and increase susceptibility to infections. Obesity is associated with a state of aberrant immune activity and increasing risk for associated inflammatory diseases, including atherosclerosis, diabetes, airway inflammation, and fatty liver disease. Thus, optimal nutritional and metabolic homeostasis is an important part of appropriate immune function and good health.
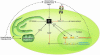
Figure 2
Lipids and inflammatory mediators: integration of metabolic and immune responses in adipocytes and macrophages through shared mechanisms. Under normal conditions, adipocytes store lipids and regulate metabolic homeostasis, and macrophages function in the inflammatory response, although each cell type has the capacity to perform both functions. In obesity, adipose tissue becomes inflamed, both via infiltration of adipose tissue by macrophages and as a result of adipocytes themselves becoming producers of inflammatory cytokines. Inflammation of adipose tissue is a crucial step in the development of peripheral insulin resistance. In addition, in proatherosclerotic conditions such as obesity and dyslipidemia, macrophages accumulate lipid to become foam cells. Adipocytes and macrophages share common features such as expression of cytokines, FABPs, nuclear hormone receptors, and many other factors. As evidenced by genetic loss-of-function models, adipocyte/macrophage FABPs modulate both lipid accumulation in adipocytes and cholesterol accumulation in macrophages, as well as the development of insulin resistance and atherosclerosis. PPARγ and LXR pathways oppose inflammation and promote cholesterol efflux from macrophages and lipid storage in adipocytes.
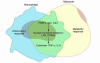
Figure 3
Nutrient and pathogen sensing or response systems have important overlapping features, and their modulation by obesity or infection can lead to overlapping physiological outcomes. For example, the chronic inflammation of obesity leads to elevated plasma lipid levels and the development of insulin resistance, eventually resulting in fatty liver disease, atherosclerosis, and diabetes. Infection typically leads to a more transient and robust inflammatory response and short-term hyperlipidemia that aids in the resolution of the infection. In some circumstances of chronic infection, however, insulin resistance, diabetes, and atherosclerosis can result.
Figure 4
Model of overlapping metabolic and inflammatory signaling and sensing pathways in adipocytes or macrophages. Inflammatory pathways can be initiated by extracellular mediators such as cytokines and lipids or by intracellular stresses such as ER stress or excess ROS production by mitochondria. Signals from all of these mediators converge on inflammatory signaling pathways, including the kinases JNK and IKK. These pathways lead to the production of additional inflammatory mediators through transcriptional regulation as well as to the direct inhibition of insulin signaling. Other pathways such as those mediated through the SOCS proteins and iNOS are also involved in inflammation-mediated inhibition of insulin action. Opposing the inflammatory pathways are transcription factors from the PPAR and LXR families, which promote nutrient transport and metabolism and antagonize inflammatory activity. More proximal regulation is provided by FABPs, which likely sequester ligands of these transcription factors, thus promoting a more inflammatory environment. The absence of FABPs is antiinflammatory. The cell must strike a balance between metabolism and inflammation. In conditions of overnutrition, this becomes a particular challenge, as the very processes required for response to nutrients and nutrient utilization, such as mitochondrial oxidative metabolism and increasing protein synthesis in the ER, can induce the inflammatory response. IR, insulin receptor.
References
- Khovidhunkit W, et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host [review] J. Lipid Res. 2004;45:1169–1196. - PubMed
- Blackburn GL. Pasteur’s Quadrant and malnutrition. Nature. 2001;409:397–401. - PubMed
- Cummings DE, Schwartz MW. Genetics and pathophysiology of human obesity. Annu. Rev. Med. 2003;54:453–471. - PubMed
- Hotamisligil, G.S. 2004. Inflammation, TNFalpha, and insulin resistance. In Diabetes mellitus: a fundamental and clinical text. D.T.S. LeRoith and J.M. Olefsky, editors. 3rd edition. Lippincott, Williams and Wilkins. New York, New York, USA. 953–962.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical