The role of cerebral amyloid beta accumulation in common forms of Alzheimer disease - PubMed (original) (raw)
Review
The role of cerebral amyloid beta accumulation in common forms of Alzheimer disease
Sam Gandy. J Clin Invest. 2005 May.
Abstract
For approximately 80 years following Alzheimer's description of the disease that bears his name, a gulf divided researchers who believed that extracellular deposits of the amyloid beta (Abeta) peptide were pathogenic from those who believed that the deposits were secondary detritus. Since 1990, the discoveries of missense mutations in the Abeta peptide precursor (APP) and the APP-cleaving enzyme presenilin 1 (PS1) have enabled much progress in understanding the molecular, cellular, and tissue pathology of the aggregates that accumulate in the interstices of the brains of patients with autosomal dominant familial Alzheimer disease (AD). Clarification of the molecular basis of common forms of AD has been more elusive. The central questions in common AD focus on whether cerebral and cerebrovascular Abeta accumulation is (a) a final neurotoxic pathway, common to all forms of AD; (b) a toxic by-product of an independent primary metabolic lesion that, by itself, is also neurotoxic; or (c) an inert by-product of an independent primary neurotoxic reaction. Antiamyloid medications are entering clinical trials so that researchers can evaluate whether abolition of cerebral amyloidosis can mitigate, treat, or prevent the dementia associated with common forms of AD. Successful development of antiamyloid medications is critical for elucidating the role of Abeta in common AD.
Figures
Figure 1
Different assembly (biophysical) states of Aβ. The assembled forms obtained from incubation of synthetic Aβ are highly sensitive to preparation and incubation. Widely differing proportions of insoluble fibrils (A), soluble PFs (B), and oligomers (also known as ADDLs) are revealed by atomic force microscopy. Typical PF and fibril preparations contain varying levels of small globular molecules, putatively Aβ oligomers (ADDLs). ADDL preparations (C) initiated from monomeric dimethyl sulfoxide stock solutions are fibril- and PF-free and uniquely comprise oligomers. Scale bars: 200 nm. Figure reproduced with permission from Trends in Neurosciences (8).
Figure 2
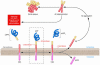
APP processing and Aβ accumulation. Mature APP (center, inside dashed box) is metabolized by 2 competing pathways, the α-secretase pathway that generates sAPPα and C83 (also known as CTFα; left) and the β-secretase pathway that generates sAPPβ and C99 (right). Some β-secretase cleavage is displaced by 10 amino acid residues and generates sAPPβ′ and C89 (see Figure 4). All carboxyterminal fragments (C83, C99, and C89) are substrates for γ-secretase, generating the APP intracellular domain (AICD) and, respectively, the secreted peptides p3 (not shown), Aβ (right), and Glu11 Aβ (see Figure 4). Aβ aggregates into small multimers (dimers, trimers, etc.) known as oligomers. Oligomers appear to be the most potent neurotoxins, while the end stage senile plaque is relatively inert.
Figure 3
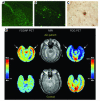
Amyloid plaque–forming transgenic mice and positron emission tomography (PET) scans of amyloid plaque load in normal human subjects and subjects with AD. (A) Thioflavin staining of subiculum of control mouse, aged 14 months. Fluorescence is nonspecific and cellular. Magnification, ×20. (B) Thioflavin staining of littermate, mutant APP X mutant PS mouse, demonstrating thioflavin-positive amyloid plaques. Magnification, ×20. (C) Immunostaining of amyloid plaque from cortex from same mouse as in B. Magnification, ×40. Figures courtesy of Michelle Ehrlich (Thomas Jefferson University). (D) [18F]FDDNP PET scan (to examine amyloid plaque and NFT load), MRI, and fluoro-deoxy-glucose (FDG) PET (to examine glucose metabolism) images of a subject with AD and a control normal subject. The [18F]FDDNP and FDG (summed) images are coregistered to their respective MRI images. Areas of FDG hypometabolism (blue) are matched with the localization of amyloid plaques and NFTs as visualized by [18F]FDDNP binding. The color bar represents the scaling of the [18F]FDDNP and FDG images. FDDNP, 2-(1-[6-[(2-[18F]fluoroethyl)(methyl)amino]-2-naphthyl]ethylidene)malononitrile; max, maximum; min, minimum. Figure reproduced with permission from the American Journal of Geriatric Psychiatry (62).
Figure 4
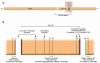
(A) Structure and topology of APP. (B) The fine structure around the Aβ domain, secretase cleavage sites, and locations of some selected familial AD missense mutations. Figure modified with permission from Trends in Endocrinology and Metabolism: TEM (15).
Figure 5
Topology of the 4 components that comprise the high molecular weight γ-secretase complex. Black bar represents the cleavage site for processing of the zymogen form of PS1 into the amino and carboxyterminal fragments that self associate and form the active enzyme. Figure modified from an image courtesy of Jan Naslund (Karolinska Institute, Stockholm, Sweden).
Figure 6
Intramembranous PS cleavage sites on 5 representative γ-secretase substrates. Figure modified with permission from Nature Reviews Molecular Cell Biology (61).
Figure 7
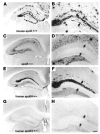
APOE isoform–specific regulation of Aβ plaque burden. Aβ plaque load is highest in the hippocampi of mice expressing murine apoE (A, magnified in B) and lowest in APOE knockout animals (C, magnified in D). Plaque load is moderate in the hippocampi of human APOE ε4–expressing mice (E, magnified in F) as compared to murine apoE–expressing mice. Plaque load in the hippocampi of human APOE ε4–expressing mice is greater than plaque load in the hippocampi from human APOE ε3–expressing mice (G, magnified in H). Amyloid deposits in the dentate gyrus, indicated by arrows, never develop in the absence of apoE. Scale bar: 150 μm (A, C, E, and G) and 60 μm (B, D, F, and H). Figure modified with permission from Proceedings of the National Academy of Sciences of the United States of America (40).
Figure 8
Comparison of the immunocytochemical distributions of APP (A and B) and PS1 (C and D) in control (A and C) and thiamine-deficient (B and D) mouse brain. Note the accumulation of APP immunoreactivity in a dystrophic neuritic cluster during thiamine deficiency (B). Arrow shows APP accumulation in an abnormal neurite arising from the neuritic cluster. PS1-immunostained sections adjacent to A and B reveal no accumulation of PS1 immunoreactivity in abnormal structures during thiamine deficiency except in areas of severe cell loss, in which the immunostaining is pale (D). Scale bar: 25 μm. Figure reproduced with permission from American Journal of Pathology (50).
Figure 9
Possible Aβ-dependent and Aβ-independent mechanisms of neurotoxicity. In this model, 1 route toward neurotoxicity begins with either APP mutations or PS1 mutations (top center of figure). The pathway toward toxicity flows downward via elevated Aβ42 levels and elevated oligomer levels. An alternative pathway is shown on the right side of the figure, whereby superoxide (O2•–) or hydrogen peroxide (H2O2) oxidize lipids such as prostaglandins, forming F2α-isoprostanes. Both H2O2 and F2α-isoprostanes are known to accelerate Aβ aggregation and presumably its oligomerization. This author proposes that toxicity in this pathway occurs both directly from reactive oxygen species (H2O2 and O2•–) and via acceleration of Aβ oligomerization by these reactive oxygen species.
Similar articles
- Amyloid-beta protein clearance and degradation (ABCD) pathways and their role in Alzheimer's disease.
Baranello RJ, Bharani KL, Padmaraju V, Chopra N, Lahiri DK, Greig NH, Pappolla MA, Sambamurti K. Baranello RJ, et al. Curr Alzheimer Res. 2015;12(1):32-46. doi: 10.2174/1567205012666141218140953. Curr Alzheimer Res. 2015. PMID: 25523424 Free PMC article. - Regulation of Alzheimer beta-amyloid precursor trafficking and metabolism.
Gandy S, Petanceska S. Gandy S, et al. Biochim Biophys Acta. 2000 Jul 26;1502(1):44-52. doi: 10.1016/s0925-4439(00)00031-4. Biochim Biophys Acta. 2000. PMID: 10899430 Review. - Loss of LR11/SORLA enhances early pathology in a mouse model of amyloidosis: evidence for a proximal role in Alzheimer's disease.
Dodson SE, Andersen OM, Karmali V, Fritz JJ, Cheng D, Peng J, Levey AI, Willnow TE, Lah JJ. Dodson SE, et al. J Neurosci. 2008 Nov 26;28(48):12877-86. doi: 10.1523/JNEUROSCI.4582-08.2008. J Neurosci. 2008. PMID: 19036982 Free PMC article. - Molecular pathogenesis of apolipoprotein E-mediated amyloidosis in late-onset Alzheimer's disease.
Tomiyama T, Corder EH, Mori H. Tomiyama T, et al. Cell Mol Life Sci. 1999 Oct 15;56(3-4):268-79. doi: 10.1007/s000180050428. Cell Mol Life Sci. 1999. PMID: 11212354 Free PMC article. Review. - Alzheimer's disease.
De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV. De-Paula VJ, et al. Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
- Emerging complexity of the HuD/ELAVl4 gene; implications for neuronal development, function, and dysfunction.
Bronicki LM, Jasmin BJ. Bronicki LM, et al. RNA. 2013 Aug;19(8):1019-37. doi: 10.1261/rna.039164.113. RNA. 2013. PMID: 23861535 Free PMC article. Review. - Evidence that an APOE ε4 'double whammy' increases risk for Alzheimer's disease.
Caesar I, Gandy S. Caesar I, et al. BMC Med. 2012 Apr 13;10:36. doi: 10.1186/1741-7015-10-36. BMC Med. 2012. PMID: 22502767 Free PMC article. - Etiology and pathogenesis of late-onset Alzheimer's disease.
Balin BJ, Hudson AP. Balin BJ, et al. Curr Allergy Asthma Rep. 2014 Mar;14(3):417. doi: 10.1007/s11882-013-0417-1. Curr Allergy Asthma Rep. 2014. PMID: 24429902 Review. - Lessons learned in the use of volumetric MRI in therapeutic trials in Alzheimer's disease: the ALZHEMED (Tramiprosate) experience.
Saumier D, Aisen PS, Gauthier S, Vellas B, Ferris SH, Duong A, Suhy J, Oh J, Lau W, Garceau D, Haine D, Sampalis J. Saumier D, et al. J Nutr Health Aging. 2009 Apr;13(4):370-2. doi: 10.1007/s12603-009-0047-4. J Nutr Health Aging. 2009. PMID: 19300884 Clinical Trial. No abstract available. - Enhancement of Amyloid β1-43 Production in the Lens Epithelium of Japanese Type 2 Diabetic Patients.
Mano Y, Otake H, Shibata T, Kubo E, Sasaki H, Nagai N. Mano Y, et al. Biomedicines. 2020 Apr 13;8(4):87. doi: 10.3390/biomedicines8040087. Biomedicines. 2020. PMID: 32294928 Free PMC article.
References
- Mitchell TW, et al. Parahippocampal tau pathology in healthy aging, mild cognitive impairment, and early Alzheimer’s disease. Ann. Neurol. 2002;51:182–189. - PubMed
- Alford MF, Masliah E, Hansen LA, Terry RD. A simple dot-immunobinding assay for quantification of synaptophysin-like immunoreactivity in human brain. J. Histochem. Cytochem. 1994;42:283–287. - PubMed
- Virchow R. Zur cellulosefrage. Virchows Arch. Pathol. Anat. Physiol. 1854;6:416–426.
- Glenner GG, Terry WD. Characterization of amyloid. Annu. Rev. Med. 1974;25:131–135. - PubMed
- Glenner GG, Wong CW. Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem. Biophys. Res. Commun. 1984;122:1131–1135. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical