Virulence and the environment: a novel role for Vibrio cholerae toxin-coregulated pili in biofilm formation on chitin - PubMed (original) (raw)
Virulence and the environment: a novel role for Vibrio cholerae toxin-coregulated pili in biofilm formation on chitin
Gemma Reguera et al. J Bacteriol. 2005 May.
Abstract
The toxin-coregulated pilus (TCP) of Vibrio cholerae is required for intestinal colonization and cholera toxin acquisition. Here we report that TCP mediates bacterial interactions required for biofilm differentiation on chitinaceous surfaces. We also show that undifferentiated TCP- biofilms have reduced ecological fitness and, thus, that chitin colonization may represent an ecological setting outside the host in which selection for a host colonization factor may take place.
Figures
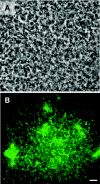
FIG. 1.
Scanning electron microscopy of WT and TCP− mutant biofilms at 48, 72, and 96 h. The arrow points at microcolonies of the WT strain formed during the development of WT biofilms on the surface of the squid pen. TCP− biofilms remained undifferentiated. Bars, 10 μm.
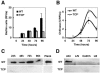
FIG. 2.
Colonization of squid pens by a WT and a TCP− mutant strain of V. cholerae. (A) Biofilm development over a 96-h period. Biofilm biomass was estimated by measuring the fluorescence from _gfp_-tagged cells (expressed as relative fluorescence units [RFUs]). (B) Chitinase activity associated with biofilm cells of the WT and TCP− mutant strains during the colonization of squid pens. Specific activities of units of chitinase activity (U, described as the amount of enzyme that released 1 pmol of 4MU per min under the assay conditions) per RFU are shown. (C) Immunodetection of TcpA, the TCP pilin subunit, in biofilm cells in the course of 96 h and in planktonic cells at 24 h (Plank). (D) Immunodetection of TcpA in cultures grown under TCP-inducing conditions (AKI medium) or grown for 24 h in the absence of a chitinous surface in NG2 mineral medium with lactate and NH4Cl (L/N) or with colloidal chitin (CollCh), and in LB medium.
FIG. 3.
Mixed biofilms of WT and TCP− mutant strains. (A) Phase-contrast micrograph showing the differentiated biofilms developed on the surface of a squid pen by a 50:50-mixed culture of a WT and a _gfp_-tagged TCP− mutant strain over a 96-h period. (B) Fluorescence micrograph placed over the micrograph in panel A showing the uniform distribution of the _gfp_-tagged TCP− mutant strain within the mixed biofilms. Bars, 10 μm.
FIG. 4.
Effect of SDS treatment on WT and TCP− mutant biofilms on a squid pen. Three-dimensional side views, generated by confocal scanning laser microscopy, of SDS-treated (+ SDS) or untreated (− SDS) biofilms of the WT and TCP mutant strains of V. cholerae showing the detachment of most cells from the undifferentiated TCP− biofilm after SDS treatment are presented; the structured WT biofilms remained unaffected. The substratum (squid pen) is located at the bottom of the images. Bars, 50 μm.
Similar articles
- A role for the mannose-sensitive hemagglutinin in biofilm formation by Vibrio cholerae El Tor.
Watnick PI, Fullner KJ, Kolter R. Watnick PI, et al. J Bacteriol. 1999 Jun;181(11):3606-9. doi: 10.1128/JB.181.11.3606-3609.1999. J Bacteriol. 1999. PMID: 10348878 Free PMC article. - Protection and attachment of Vibrio cholerae mediated by the toxin-coregulated pilus in the infant mouse model.
Krebs SJ, Taylor RK. Krebs SJ, et al. J Bacteriol. 2011 Oct;193(19):5260-70. doi: 10.1128/JB.00378-11. Epub 2011 Jul 29. J Bacteriol. 2011. PMID: 21804008 Free PMC article. - The role of toxin-coregulated pili in the pathogenesis of Vibrio cholerae O1 El Tor.
Attridge SR, Voss E, Manning PA. Attridge SR, et al. Microb Pathog. 1993 Dec;15(6):421-31. doi: 10.1006/mpat.1993.1091. Microb Pathog. 1993. PMID: 7911968 - Regulation of virulence in Vibrio cholerae.
Klose KE. Klose KE. Int J Med Microbiol. 2001 May;291(2):81-8. doi: 10.1078/1438-4221-00104. Int J Med Microbiol. 2001. PMID: 11437342 Review.
Cited by
- Quorum regulated latent environmental cells of toxigenic Vibrio cholerae and their role in cholera outbreaks.
Faruque SN, Yamasaki S, Faruque SM. Faruque SN, et al. Gut Pathog. 2024 Sep 29;16(1):52. doi: 10.1186/s13099-024-00647-3. Gut Pathog. 2024. PMID: 39343919 Free PMC article. Review. - The role of filamentous matrix molecules in shaping the architecture and emergent properties of bacterial biofilms.
Böhning J, Tarafder AK, Bharat TAM. Böhning J, et al. Biochem J. 2024 Feb 21;481(4):245-263. doi: 10.1042/BCJ20210301. Biochem J. 2024. PMID: 38358118 Free PMC article. Review. - The Role of Nutrients and Nutritional Signals in the Pathogenesis of Vibrio cholerae.
McDonald ND, Rosenberger JR, Almagro-Moreno S, Boyd EF. McDonald ND, et al. Adv Exp Med Biol. 2023;1404:195-211. doi: 10.1007/978-3-031-22997-8_10. Adv Exp Med Biol. 2023. PMID: 36792877 - Cholera Dynamics and the Emergence of Pandemic Vibrio cholerae.
Balasubramanian D, López-Pérez M, Almagro-Moreno S. Balasubramanian D, et al. Adv Exp Med Biol. 2023;1404:127-147. doi: 10.1007/978-3-031-22997-8_7. Adv Exp Med Biol. 2023. PMID: 36792874 - New Insights into Vibrio cholerae Biofilms from Molecular Biophysics to Microbial Ecology.
Tai JB, Ferrell MJ, Yan J, Waters CM. Tai JB, et al. Adv Exp Med Biol. 2023;1404:17-39. doi: 10.1007/978-3-031-22997-8_2. Adv Exp Med Biol. 2023. PMID: 36792869 Free PMC article.
References
- Colwell, R. R., J. Kaper, and S. W. Joseph. 1977. Vibrio cholerae, Vibrio parahaemolyticus, and other vibrios: occurrence and distribution in Chesapeake Bay. Science 198:394-396. - PubMed
- Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources