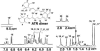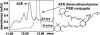Isolation and characterization of a retinal pigment epithelial cell fluorophore: an all-trans-retinal dimer conjugate - PubMed (original) (raw)
Comparative Study
. 2005 May 17;102(20):7091-6.
doi: 10.1073/pnas.0501266102. Epub 2005 May 3.
Affiliations
- PMID: 15870200
- PMCID: PMC1129110
- DOI: 10.1073/pnas.0501266102
Comparative Study
Isolation and characterization of a retinal pigment epithelial cell fluorophore: an all-trans-retinal dimer conjugate
Nathan E Fishkin et al. Proc Natl Acad Sci U S A. 2005.
Abstract
Several lines of investigation suggest that the nondegradable fluorophores that accumulate as lipofuscin in retinal pigment epithelium (RPE) cells contribute to the etiology of macular degeneration. Despite evidence that much of this fluorescent material may originate as inadvertent products of the retinoid cycle, the enzymatic pathway by which the 11-cis-retinal chromophore of rhodopsin is generated, the only fluorophores of the RPE to be characterized as yet have been A2E and its isomers. Here, we report the isolation and structural characterization of an additional RPE lipofuscin fluorophore that originates as a condensation product of two molecules of all-trans-retinal (ATR) dimer and forms a protonated Schiff base conjugate with phosphatidylethanolamine (PE), the latter conjugate (ATR dimer-PE) having UV-visible absorbance maxima at 285 and 506 nm. ATR dimer was found to form natively in bleached rod outer segments in vitro and when rod outer segments were incubated with ATR. HPLC analysis of eye-cups that included RPE and isolated neural retina from Abcr-/- mice and RPE isolated from human donor eyes revealed the presence of a pigment with the same UV-visible absorbance and retention time as synthetic ATR dimer-PE conjugate. Evidence that ATR dimer undergoes a photooxidation process involving the addition of oxygens at double bonds as well as an aromatic demethylation also may indicate a role for this molecule, or its derivatives, in the photoreactivity of RPE lipofuscin.
Figures
Fig. 1.
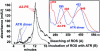
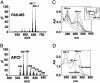
Reverse-phase HPLC profile of extracts of bovine ROS that had been bleached to release endogenous ATR (trace A) or that had been incubated with exogenous ATR (trace B). Absorbance was detected at 450 nm. A2-PE and ATR dimer were identified by coinjection of synthetic samples of these pigments. (Upper Right) UV-visible spectra of A2-PE and ATR dimer in acetonitrile/water are shown.
Fig. 2.
1H NMR spectrum (400 MHz; CDCl3) of the pigment in ATR-treated ROS that was isolated by using reverse-phase HPLC at a retention time of 6.95 min. Two sets of _gem_-dimethyl peaks on C1 and C1′ were immediately suggestive of a dimerized retinal product and verified the structure as that of ATR dimer. The peaks are assigned based on the numbering shown with the structure. X, solvent impurities.
Fig. 3.
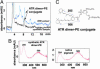
Formation of an ATR dimer-PE conjugate. (A) Normal-phase HPLC (0.1% AcOH buffer, 500-nm detection) profiles of synthetic ATR dimer-PE conjugate (synthetic sample) and a pigment extracted from RPE eyecups of Abcr -/- mice (RPE extract). mAU, milliabsorbance unit. (B) UV-visible spectra of synthetic ATR dimer-PE conjugate (Left) and the native 506-nm pigment isolated from Abcr -/- mice (Right). (C) Structure of ATR dimer-PE conjugate. The absorbance spectrum of this pigment is due to 295- and 506-nm chromophores.
Fig. 4.
Reverse-phase HPLC profile of ATR dimer-ethanolamine 5 min after exposure to 100 mM HCl and again 24 h later. The peak corresponding to ATR dimer-ethanolamine conjugate was depleted and replaced by a peak with a retention time and UV spectrum consistent with A2E.
Fig. 5.
Identification of ATR dimer-PE conjugate in neural retina. (Upper) Normal-phase HPLC of synthetic ATR dimer-PE conjugate and chloroform/methanol extract of neural retina from Abcr -/- mice. (Lower) UV-visible spectra of synthetic ATR dimer-PE conjugate (Left; retention time, 10.6 min) and corresponding native pigment from neural retina (Right; retention time, 10.6 min).
Fig. 6.
The SB nitrogen in ATR dimer-PE conjugate is protonated. The UV-visible spectrum of native 506-nm pigment isolated from eyecups of Abcr -/- mice and analyzed by normal-phase HPLC without acetic acid buffer is shown. Raising the pH leads to the appearance of an additional peak at 418 nm due to deprotonation of the SB. The presence of both the 418- and 506-nm peaks indicates an equilibrium between the SB and PSB forms of the pigment.
Fig. 7.
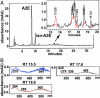
Detection of ATR dimer-PE conjugate in human RPE cells. (A) Reverse-phase HPLC analysis of extracts of isolated RPE pooled from six human eyes. Monitoring was at 500 nm. (B) UV-visible spectra of peaks at retention times (RT) 15.5, 17.6, and 18.6 min exhibit absorbances centered at 500 nm.
Fig. 8.
Bleaching behavior of ATR dimer in chloroform and water. ATR dimer (100 μM) in water (A and C) or chloroform (B and D) was exposed to 2 min of blue light (430 nm, 60 W, 10 cm) and then analyzed by using MS (A and B). FAB, fast atom bombardment; APCI, atmospheric pressure chemical ionization. UV-visible spectra (C and D) were obtained after successive 10-sec bleaches in water (C) and after the indicated bleaching times in chloroform (D). C Inset shows an isosbestic point at 259 nm and a corresponding increase in absorption at 246 nm, which is consistent with the main charge transfer band of benzaldehyde. Arrows indicate the trends in absorbance change.
Fig. 9.
1H NMR analysis of irradiated ATR dimer. ATR dimer was either unirradiated (no _h_ν) or irradiated for 2, 4, or 6 min (430 nm, 0.19 mW/mm2). The presence of aromatic protons (arrows) upon blue-light exposure suggests aromatization of the central hexadiene core.
Fig. 10.
Proposed biosynthetic pathway of ATR dimer and A2E. ATR that is released from opsin upon photoisomerization of 11-_cis_-retinal reacts with PE to produce the _N_-retinyl-phosphatidylethanolamine (NRPE) SB. Both ATR dimer and A2-PE may form from the same tautomer x arising from a [1,6] H-shift of NRPE (15). Tautomer x can form an iminium ion with a second ATR according to path a, and after 6 π-aza electrocyclization and autooxidation, A2-PE is formed. Conversely, in path b, C20 of NRPE reacts with C13 of free ATR in a Michael-type addition, followed by a Mannich reaction to close the ring, and elimination of the amino group of PE, yielding the ATR dimer.
Similar articles
- The all-trans-retinal dimer series of lipofuscin pigments in retinal pigment epithelial cells in a recessive Stargardt disease model.
Kim SR, Jang YP, Jockusch S, Fishkin NE, Turro NJ, Sparrow JR. Kim SR, et al. Proc Natl Acad Sci U S A. 2007 Dec 4;104(49):19273-8. doi: 10.1073/pnas.0708714104. Epub 2007 Nov 28. Proc Natl Acad Sci U S A. 2007. PMID: 18048333 Free PMC article. - Novel lipofuscin bisretinoids prominent in human retina and in a model of recessive Stargardt disease.
Wu Y, Fishkin NE, Pande A, Pande J, Sparrow JR. Wu Y, et al. J Biol Chem. 2009 Jul 24;284(30):20155-66. doi: 10.1074/jbc.M109.021345. Epub 2009 May 28. J Biol Chem. 2009. PMID: 19478335 Free PMC article. - Characterization of peroxy-A2E and furan-A2E photooxidation products and detection in human and mouse retinal pigment epithelial cell lipofuscin.
Jang YP, Matsuda H, Itagaki Y, Nakanishi K, Sparrow JR. Jang YP, et al. J Biol Chem. 2005 Dec 2;280(48):39732-9. doi: 10.1074/jbc.M504933200. Epub 2005 Sep 26. J Biol Chem. 2005. PMID: 16186115 - The 11-cis Retinal Origins of Lipofuscin in the Retina.
Adler L 4th, Boyer NP, Chen C, Ablonczy Z, Crouch RK, Koutalos Y. Adler L 4th, et al. Prog Mol Biol Transl Sci. 2015;134:e1-12. doi: 10.1016/bs.pmbts.2015.07.022. Prog Mol Biol Transl Sci. 2015. PMID: 26310175 Review. - A2E, a byproduct of the visual cycle.
Sparrow JR, Fishkin N, Zhou J, Cai B, Jang YP, Krane S, Itagaki Y, Nakanishi K. Sparrow JR, et al. Vision Res. 2003 Dec;43(28):2983-90. doi: 10.1016/s0042-6989(03)00475-9. Vision Res. 2003. PMID: 14611934 Review.
Cited by
- Fundus autofluorescence and the bisretinoids of retina.
Sparrow JR, Wu Y, Nagasaki T, Yoon KD, Yamamoto K, Zhou J. Sparrow JR, et al. Photochem Photobiol Sci. 2010 Nov;9(11):1480-9. doi: 10.1039/c0pp00207k. Epub 2010 Sep 23. Photochem Photobiol Sci. 2010. PMID: 20862444 Free PMC article. - Altered retinoid homeostasis catalyzed by a nicotine metabolite: implications in macular degeneration and normal development.
Brogan AP, Dickerson TJ, Boldt GE, Janda KD. Brogan AP, et al. Proc Natl Acad Sci U S A. 2005 Jul 26;102(30):10433-8. doi: 10.1073/pnas.0504721102. Epub 2005 Jul 13. Proc Natl Acad Sci U S A. 2005. PMID: 16014706 Free PMC article. - [Comparison of parameters of time-resolved autofluorescence between healthy subjects and patients suffering from early AMD].
Schweitzer D, Quick S, Schenke S, Klemm M, Gehlert S, Hammer M, Jentsch S, Fischer J. Schweitzer D, et al. Ophthalmologe. 2009 Aug;106(8):714-22. doi: 10.1007/s00347-009-1975-4. Ophthalmologe. 2009. PMID: 19588156 German. - Novel bisretinoids of human retina are lyso alkyl ether glycerophosphoethanolamine-bearing A2PE species.
Kim HJ, Sparrow JR. Kim HJ, et al. J Lipid Res. 2018 Sep;59(9):1620-1629. doi: 10.1194/jlr.M084459. Epub 2018 Jul 9. J Lipid Res. 2018. PMID: 29986955 Free PMC article. - The reduction of retinal autofluorescence caused by light exposure.
Morgan JI, Hunter JJ, Merigan WH, Williams DR. Morgan JI, et al. Invest Ophthalmol Vis Sci. 2009 Dec;50(12):6015-22. doi: 10.1167/iovs.09-3643. Epub 2009 Jul 23. Invest Ophthalmol Vis Sci. 2009. PMID: 19628734 Free PMC article.
References
- Cuervo, A. M. & Dice, J. R. (2000) Exp. Gerontol. 35, 119-131. - PubMed
- Katz, M. L., Drea, C. M., Eldred, G. E., Hess, H. H. & Robison, W. G., Jr. (1986) Exp. Eye. Res. 43, 561-573. - PubMed
- Katz, M. L., Drea, C. M. & Robison, W. G., Jr. (1986) Mech. Ageing Dev. 35, 291-305. - PubMed
- Katz, M. L. & Redmond, T. M. (2001) Invest. Ophthalmol. Visual Sci. 42, 3023-3030. - PubMed
- Wing, G. L., Blanchard, G. C. & Weiter, J. J. (1978) Invest. Ophthalmol. Visual Sci. 17, 601-607. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY013435/EY/NEI NIH HHS/United States
- R01 EY012951/EY/NEI NIH HHS/United States
- EY12951/EY/NEI NIH HHS/United States
- GM34509/GM/NIGMS NIH HHS/United States
- EY13435/EY/NEI NIH HHS/United States
- R01 GM034509/GM/NIGMS NIH HHS/United States
- EY139933/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous