Kinetics of exocytosis is faster in cones than in rods - PubMed (original) (raw)
Comparative Study
Kinetics of exocytosis is faster in cones than in rods
Katalin Rabl et al. J Neurosci. 2005.
Erratum in
- J Neurosci. 2005 Nov 2;25(44):table of contents
Abstract
Cone-driven responses of second-order retinal neurons are considerably faster than rod-driven responses. We examined whether differences in the kinetics of synaptic transmitter release from rods and cones may contribute to differences in postsynaptic response kinetics. Exocytosis from rods and cones was triggered by membrane depolarization and monitored in two ways: (1) by measuring EPSCs evoked in second-order neurons by depolarizing steps applied to presynaptic rods or cones during simultaneous paired whole-cell recordings or (2) by direct measurements of exocytotic increases in membrane capacitance. The kinetics of release was assessed by varying the length of the depolarizing test step. Both measures of release revealed two kinetic components to the increase in exocytosis as a function of the duration of a step depolarization. In addition to slow sustained components in both cell types, the initial fast component of exocytosis had a time constant of <5 ms in cones, >10-fold faster than that of rods. Rod/cone differences in the kinetics of release were substantiated by a linear correlation between depolarization-evoked capacitance increases and EPSC charge transfer. Experiments on isolated rods indicate that the slower kinetics of exocytosis from rods was not a result of rod-rod coupling. The initial rapid release of vesicles from cones can shape the postsynaptic response and may contribute to the faster responses of cone-driven cells observed at light offset.
Figures
Figure 1.
Depolarizing steps (-70 to -10 mV) applied to rods or cones evoked transient EPSCs in simultaneously recorded OFF bipolar cells. Cyclothiazide (0.1 m
m
) was added to inhibit AMPA receptor desensitization. A, EPSCs evoked by steps of 10, 100, and 1000 ms duration. B, Overlay of EPSCs evoked by 100 ms steps in rod-driven (thin trace) and cone-driven (thick trace) OFF bipolar cells shows that cone-driven EPSCs exhibited faster onset and offset than rod-driven EPSCs.
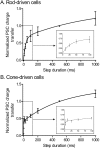
Figure 2.
There are two kinetic components to EPSCs evoked by depolarizing steps applied to rods and cones. Increasing the duration (10 to 1000 ms) of depolarizing steps (-70 to -10 mV) applied to rods or cones caused a biexponential increase in the EPSC charge transfer measured in OFF bipolar and horizontal cells during paired recordings from retinal slices. Data from rod-driven cells are shown in A, and data from cone-driven cells are shown in B. Fits to short-duration steps are expanded in the in sets. Postsynaptic recordings were obtained from both OFF bipolar cells (rod driven, n = 5; cone driven, n = 6) and horizontal cells (rod driven, n = 3; cone driven, n = 6) in the presence of cyclothiazide (0.1 m
m
). Data from six of the rod-driven cells were used for a similar comparison by Thoreson et al. (2004). No differences were detected between response/duration relationships in OFF bipolar and horizontal cells when synaptically stimulated by the same type of photoreceptor. Charge transfer during the EPSC was integrated and normalized to the charge transfer evoked by a 500 ms depolarizing step. Data were fit with dual-exponential functions (rods, τ1 = 27.5 ms, τ2 = 954 ms; cones, τ1 = 2.4 ms, τ2 = 570 ms). Error bars represent SEM.
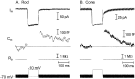
Figure 3.
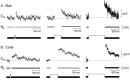
Membrane depolarization triggered exocytosis from rods and cones in a retinal slice. Traces show membrane current (_I_m), membrane capacitance (_C_m), access resistance (_R_a), and the stimulus protocol. Capacitance was monitored in a rod (A) and a cone (B) with a phase-lock amplifier while sinusoidally varying the holding potential (600 Hz, ±15 mV) about the mean holding potential of -70 mV. Phase tracking was gated out during the depolarizing test step to -10 mV (100 ms) and for 3 ms after the step. Passive membrane resistances of 500 MΩ in the rod and 800 MΩ in the cone were leak subtracted from the current traces.
Figure 4.
Tail currents could be evoked without altering capacitance measurements. A, Little or no capacitance change was observed when the membrane resistance in a model cone cell was manually reduced from 800 to 500 MΩ to simulate the large conductance change accompanying a tail current. Capacitance was monitored using the phase-tracking feature of the Optopatch amplifer as described in Materials and Methods. B, An example from a rod photoreceptor showing that tail currents could be evoked in cells without accompanying capacitance changes. In this experiment, niflumic acid was omitted to permit the generation of tail currents. Early in the recording from this rod, the depolarizing test step (200 ms, -70 to -10 mV) evoked an inward tail current at the end of the step (top left) and a large capacitance increase (bottom left). Application of the same test step 15 min later evoked similar inward tail currents (top right) but no capacitance increase (bottom right).
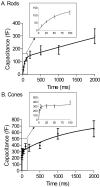
Figure 6.
There are two kinetic components to exocytosis from rods (A) and cones (B). Depolarization-evoked capacitance increases were plotted as a function of step duration. Capacitance/duration relationships were fit with dual-exponential functions. Fits to short-duration steps are expanded in the insets. Rods, τ1 = 46 ms, Amplitude1 (A1) = 132 fF, τ2 = 14 s, Amplitude2 (A2) = 1170 fF; cones, τ1 = 2.7 ms, A1 = 306 fF, τ2 = 1.4 s, A2 = 474 fF. Sample sizes: rods, 10 ms, n = 9; 25 ms, n = 11; 50 ms, n = 10; 100 ms, n = 12; 200 ms, n = 11; 500 ms, n = 10; 1000 ms, n = 10; 2000 ms, n = 7; cones, 3 ms, n = 9; 5 ms, n = 5; 10 ms, n = 16; 25 ms, n = 14; 50 ms, n = 13; 100 ms, n = 20; 200 ms, n = 12; 500 ms, n = 11; 1000 ms, n = 11; 2000 ms, n = 12. Error bars represent SEM.
Figure 7.
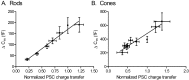
Capacitance (_C_m) changes evoked in rods (A) or cones (B) by depolarizing steps (-70 to -10 mV) of varying duration (3-1000 ms in rods, 3-2000 ms in cones) are linearly correlated with the integrated charge transfer of EPSCs in OFF bipolar and horizontal cells (rods, _r_2 = 0.95; cones, _r_2 = 0.91). Error bars represent SEM.
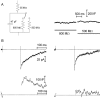
Figure 5.
Increasing the duration of a depolarizing step increased the capacitance jump in both rods and cones. Top traces show the capacitance (_C_m) increases evoked in a rod (A) and a cone (B) by 10, 100, and 1000 ms depolarizing steps (-70 to -10 mV). No significant changes in access resistance (middle traces; _R_a) associated with the voltage steps (bottom traces) were observed.
Similar articles
- Kinetics of synaptic transmission at ribbon synapses of rods and cones.
Thoreson WB. Thoreson WB. Mol Neurobiol. 2007 Dec;36(3):205-23. doi: 10.1007/s12035-007-0019-9. Epub 2007 Jul 10. Mol Neurobiol. 2007. PMID: 17955196 Free PMC article. Review. - Paired-pulse depression at photoreceptor synapses.
Rabl K, Cadetti L, Thoreson WB. Rabl K, et al. J Neurosci. 2006 Mar 1;26(9):2555-63. doi: 10.1523/JNEUROSCI.3667-05.2006. J Neurosci. 2006. PMID: 16510733 Free PMC article. - A comparison of release kinetics and glutamate receptor properties in shaping rod-cone differences in EPSC kinetics in the salamander retina.
Cadetti L, Tranchina D, Thoreson WB. Cadetti L, et al. J Physiol. 2005 Dec 15;569(Pt 3):773-88. doi: 10.1113/jphysiol.2005.096545. Epub 2005 Oct 13. J Physiol. 2005. PMID: 16223761 Free PMC article. - Calcium-induced calcium release in rod photoreceptor terminals boosts synaptic transmission during maintained depolarization.
Cadetti L, Bryson EJ, Ciccone CA, Rabl K, Thoreson WB. Cadetti L, et al. Eur J Neurosci. 2006 Jun;23(11):2983-90. doi: 10.1111/j.1460-9568.2006.04845.x. Eur J Neurosci. 2006. PMID: 16819987 Free PMC article. - Molecular mechanisms characterizing cone photoresponses.
Tachibanaki S, Shimauchi-Matsukawa Y, Arinobu D, Kawamura S. Tachibanaki S, et al. Photochem Photobiol. 2007 Jan-Feb;83(1):19-26. doi: 10.1562/2006-02-28-IR-823. Photochem Photobiol. 2007. PMID: 16706600 Review.
Cited by
- Regulation of synaptic transmission at the photoreceptor terminal: a novel role for the cation-chloride co-transporter NKCC1.
Shen W, Purpura LA, Li B, Nan C, Chang IJ, Ripps H. Shen W, et al. J Physiol. 2013 Jan 1;591(1):133-47. doi: 10.1113/jphysiol.2012.241042. Epub 2012 Oct 22. J Physiol. 2013. PMID: 23090945 Free PMC article. - Preparation of Horizontal Slices of Adult Mouse Retina for Electrophysiological Studies.
Feigenspan A, Babai NZ. Feigenspan A, et al. J Vis Exp. 2017 Jan 27;(119):55173. doi: 10.3791/55173. J Vis Exp. 2017. PMID: 28190066 Free PMC article. - Calmodulin enhances ribbon replenishment and shapes filtering of synaptic transmission by cone photoreceptors.
Van Hook MJ, Parmelee CM, Chen M, Cork KM, Curto C, Thoreson WB. Van Hook MJ, et al. J Gen Physiol. 2014 Nov;144(5):357-78. doi: 10.1085/jgp.201411229. Epub 2014 Oct 13. J Gen Physiol. 2014. PMID: 25311636 Free PMC article. - Kinetics of synaptic transmission at ribbon synapses of rods and cones.
Thoreson WB. Thoreson WB. Mol Neurobiol. 2007 Dec;36(3):205-23. doi: 10.1007/s12035-007-0019-9. Epub 2007 Jul 10. Mol Neurobiol. 2007. PMID: 17955196 Free PMC article. Review. - Ca2+ Diffusion through Endoplasmic Reticulum Supports Elevated Intraterminal Ca2+ Levels Needed to Sustain Synaptic Release from Rods in Darkness.
Chen M, Van Hook MJ, Thoreson WB. Chen M, et al. J Neurosci. 2015 Aug 12;35(32):11364-73. doi: 10.1523/JNEUROSCI.0754-15.2015. J Neurosci. 2015. PMID: 26269643 Free PMC article.
References
- Barnes S, Deschenes MC (1992) Contribution of Ca and Ca-activated Cl channels to regenerative depolarization and membrane bistability of cone photoreceptors. J Neurophysiol 68: 745-755. - PubMed
- Copenhagen DR, Ashmore JF, Schnapf JK (1983) Kinetics of synaptic transmission from photoreceptors to horizontal and bipolar cells in turtle retina. Vision Res 23: 363-369. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources