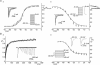Mechanisms underlying the early phase of spike frequency adaptation in mouse spinal motoneurones - PubMed (original) (raw)
Mechanisms underlying the early phase of spike frequency adaptation in mouse spinal motoneurones
G B Miles et al. J Physiol. 2005.
Abstract
Spike frequency adaptation (SFA) is a fundamental property of repetitive firing in motoneurones (MNs). Early SFA (occurring over several hundred milliseconds) is thought to be important in the initiation of muscular contraction. To date the mechanisms underlying SFA in spinal MNs remain unclear. In the present study, we used both whole-cell patch-clamp recordings of MNs in lumbar spinal cord slices prepared from motor functionally mature mice and computer modelling of spinal MNs to investigate the mechanisms underlying SFA. Pharmacological blocking agents applied during whole-cell recordings in current-clamp mode demonstrated that the medium AHP conductance (apamin), BK-type Ca2+ -dependent K+ channels (iberiotoxin), voltage-activated Ca2+ channels (CdCl2), M-current (linopirdine) and persistent Na+ currents (riluzole) are all unnecessary for SFA. Measurements of Na+ channel availability including action potential amplitude, action potential threshold and maximum depolarization rate of the action potential were found to correlate with instantaneous firing frequency suggesting that the availability of fast, inactivating Na+ channels is involved in SFA. Characterization of this Na+ conductance in voltage-clamp mode demonstrated that it undergoes slow inactivation with a time course similar to that of SFA. When experimentally measured parameters for the fast, inactivating Na+ conductance (including slow inactivation) were incorporated into a MN model, SFA could be faithfully reproduced. The removal of slow inactivation from this model was sufficient to remove SFA. These data indicate that slow inactivation of the fast, inactivating Na+ conductance is likely to be the key mechanism underlying early SFA in spinal MNs.
Figures
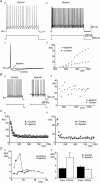
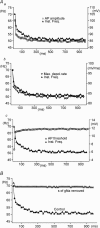
Figure 1. Apamin blocks the AHP and increases variability in steady-state firing frequency, but spike frequency adaptation (SFA) remains
Aa and b, current-clamp recordings of repetitive firing elicited by current injection (1 s duration) in control (a) and with 100 n
m
apamin (b). Ac, single action potentials evoked by brief current pulses (10 ms) showing block of the AHP by apamin (dotted line). Ad, steady-state frequency versus injected current plots showing increased excitability in the presence of apamin. Ba, steady-state firing over the last 500 ms of a 1 s current pulse in control and in the presence of apamin. Bb, plot of instantaneous firing frequency versus time for firing during the last 500 ms of a 1 s current pulse showing the greater variability in frequencies in the presence of apamin. Instantaneous frequency versus time plots showing SFA in control and in the presence of apamin during relatively fast (Ca) and slow (Cb) rates of firing (current steps eliciting similar steady-state firing frequencies were chosen for each condition). Da, SFA ratio plotted versus steady-state frequency in control and in the presence of apamin for a single MN. Db, pooled data showing that apamin significantly increases adaptation at lower steady-state firing frequencies (< 30 Hz) (n = 6).
Figure 2. BK-type Ca2+-dependent K+ channels, voltage-activated Ca2+ channels and M-currents are not required for SFA
A, instantaneous frequency versus time plots for repetitive firing elicited by single current steps. SFA remains following the application of either the BK type calcium-dependent potassium channel blocker iberiotoxin (100 n
m
), the general voltage-gated calcium channel blocker cadmium (0.5 m
m
), or the M-current blocker linopirdine (10 μ
m
). Note, current pulses eliciting similar steady-state firing frequencies were plotted for each condition.
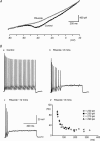
Figure 3. Block of persistent Na+ currents by riluzole inhibits repetitive firing but not SFA
A, voltage-clamp recordings in solutions designed to isolate sodium currents (see Methods for details) showing the current response to a voltage ramp from −80 to +20 mV (∼70 mV s−1). Under control conditions (dark trace) a persistent inward current is revealed. This current is blocked by the persistent sodium current blocker riluzole (10 μ
m
, light trace). B, I-clamp recordings in standard solutions showing that repetitive firing observed in control (a) is reduced (b) and eventually blocked (c) by riluzole (10 μ
m
). Bd, instantaneous frequency versus time plots showing that although repetitive firing is reduced after 8 min of riluzole application, SFA remains. The different data series represent firing in response to current injection of varying magnitude.
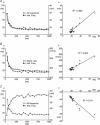
Figure 4. SFA correlates with measurements of Na+ channel availability
Aa, action potential overshoot versus time plotted with instantaneous frequency versus time for a single current pulse (duration 1 s). Ab, action potential overshoot plotted versus instantaneous frequency showing a strong positive correlation. Ba, maximum rate of action potential depolarization versus time plotted with instantaneous frequency versus time. Bb, maximum rate of action potential depolarization plotted versus instantaneous frequency showing a strong positive correlation. Ca, action potential threshold (defined as voltage at which rate of d_V_/d_t_ = 10 mV ms−1) versus time plotted with instantaneous frequency versus time. Cb, action potential threshold plotted versus instantaneous frequency showing a strong inverse correlation.
Figure 5. Activation and inactivation properties of Na+ currents in spinal MNs
Aa, peak activation curve for _I_Na fitted with a Boltzmann-type equation (_V_half=–37 mV, slope = 4.3). Each point is from averaged data (n = 5–8 cells). Ab, steady-state fast inactivation curve for _I_Na fitted with a Boltzmann-type equation (_V_half=−39 mV, slope = 7.5). Each point is from averaged data (n = 8–14). Ba, time course of recovery of _I_Na from slow inactivation measured using a two-pulse protocol (see results). The graph shows the peak current elicited by the second step (_I_2) divided by the peak current in response to the first step (_I_1) plotted versus the time between the two steps. Each point is from averaged data (n = 6). Recovery followed a bi-exponential time course with time constants of 15.7 ms and 129.2 ms. Bb, slow inactivation curve for _I_Na fitted with a Boltzmann-type equation (_V_half=−37 mV, slope = 8). Each point is from averaged data (n = 7). Insets in Aa, Ab and Bb depict the voltage protocol used and the currents elicited by protocols. Inset in B shows currents elicited by the two-pulse inactivation protocol.
Figure 6. The MN model exhibits SFA, which correlates with Na+ channel availability, and is dependent on the inclusion of slow inactivation of the Na+ conductance
Data from computer modelling showing similar results, in response to injection of a single current pulse, as those seen in MN recordings. Aa, action potential amplitude and instantaneous frequency plotted versus time during simulated current injection. Ab, maximum rate of depolarization of action potentials and instantaneous frequency plotted versus time. Ac, action potential threshold (defined as voltage at which rate of d_V_/d_t_ = 10 mV ms−1) and instantaneous frequency plotted versus time. B, instantaneous frequency versus time plots showing SFA in control conditions when all conductances are included in the model (filled triangles), and the abolishment of SFA when slow inactivation (s) of the Na+ conductance is removed (open triangles).
Similar articles
- Iberiotoxin-sensitive large conductance Ca2+ -dependent K+ (BK) channels regulate the spike configuration in the burst firing of cerebellar Purkinje neurons.
Haghdoost-Yazdi H, Janahmadi M, Behzadi G. Haghdoost-Yazdi H, et al. Brain Res. 2008 May 30;1212:1-8. doi: 10.1016/j.brainres.2008.03.030. Epub 2008 Mar 27. Brain Res. 2008. PMID: 18439989 - Development of ionic currents underlying changes in action potential waveforms in rat spinal motoneurons.
Gao BX, Ziskind-Conhaim L. Gao BX, et al. J Neurophysiol. 1998 Dec;80(6):3047-61. doi: 10.1152/jn.1998.80.6.3047. J Neurophysiol. 1998. PMID: 9862905 - Calcium-activated SK channels influence voltage-gated ion channels to determine the precision of firing in globus pallidus neurons.
Deister CA, Chan CS, Surmeier DJ, Wilson CJ. Deister CA, et al. J Neurosci. 2009 Jul 1;29(26):8452-61. doi: 10.1523/JNEUROSCI.0576-09.2009. J Neurosci. 2009. PMID: 19571136 Free PMC article. - Electrophysiological properties of guinea pig trigeminal motoneurons recorded in vitro.
Chandler SH, Hsaio CF, Inoue T, Goldberg LJ. Chandler SH, et al. J Neurophysiol. 1994 Jan;71(1):129-45. doi: 10.1152/jn.1994.71.1.129. J Neurophysiol. 1994. PMID: 7908952 - Contribution of apamin-sensitive SK channels to the firing precision but not to the slow afterhyperpolarization and spike frequency adaptation in snail neurons.
Vatanparast J, Janahmadi M. Vatanparast J, et al. Brain Res. 2009 Feb 19;1255:57-66. doi: 10.1016/j.brainres.2008.12.003. Epub 2008 Dec 11. Brain Res. 2009. PMID: 19100724
Cited by
- Retracing your footsteps: developmental insights to spinal network plasticity following injury.
Jean-Xavier C, Sharples SA, Mayr KA, Lognon AP, Whelan PJ. Jean-Xavier C, et al. J Neurophysiol. 2018 Feb 1;119(2):521-536. doi: 10.1152/jn.00575.2017. Epub 2017 Oct 25. J Neurophysiol. 2018. PMID: 29070632 Free PMC article. Review. - Sodium Pumps Mediate Activity-Dependent Changes in Mammalian Motor Networks.
Picton LD, Nascimento F, Broadhead MJ, Sillar KT, Miles GB. Picton LD, et al. J Neurosci. 2017 Jan 25;37(4):906-921. doi: 10.1523/JNEUROSCI.2005-16.2016. J Neurosci. 2017. PMID: 28123025 Free PMC article. - Differential regulation of synaptic transmission by pre- and postsynaptic SK channels in the spinal locomotor network.
Nanou E, Alpert MH, Alford S, El Manira A. Nanou E, et al. J Neurophysiol. 2013 Jun;109(12):3051-9. doi: 10.1152/jn.00067.2013. Epub 2013 Apr 3. J Neurophysiol. 2013. PMID: 23554432 Free PMC article. - Diversity of Mammalian Motoneurons and Motor Units.
Bączyk M, Manuel M, Roselli F, Zytnicki D. Bączyk M, et al. Adv Neurobiol. 2022;28:131-150. doi: 10.1007/978-3-031-07167-6_6. Adv Neurobiol. 2022. PMID: 36066824 - Diphenytoin, riluzole and lidocaine: three sodium channel blockers, with different mechanisms of action, decrease hippocampal epileptiform activity.
Diao L, Hellier JL, Uskert-Newsom J, Williams PA, Staley KJ, Yee AS. Diao L, et al. Neuropharmacology. 2013 Oct;73:48-55. doi: 10.1016/j.neuropharm.2013.04.057. Epub 2013 May 21. Neuropharmacology. 2013. PMID: 23707481 Free PMC article.
References
- Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3:331–338. - PubMed
- Baldissera F, Gustafsson B. Regulation of repetitive firing in motoneurones by the afterhyperpolarization conductance. Brain Res. 1971;30:431–434. - PubMed
- Baldissera F, Gustafsson B. Firing behaviour of a neurone model based on the afterhyperpolarization conductance time course and algebraical summation. Adaptation and steady state firing. Acta Physiol Scand. 1974;92:27–47. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous