Galectin-4 and sulfatides in apical membrane trafficking in enterocyte-like cells - PubMed (original) (raw)
. 2005 May 9;169(3):491-501.
doi: 10.1083/jcb.200407073.
Valérie Gouyer, Jean-Pierre Zanetta, Hervé Drobecq, Emmanuelle Leteurtre, Georges Grard, Odile Moreau-Hannedouche, Emmanuel Maes, Alexandre Pons, Sabine André, André Le Bivic, Hans Joachim Gabius, Aki Manninen, Kai Simons, Guillemette Huet
Affiliations
- PMID: 15883199
- PMCID: PMC2171948
- DOI: 10.1083/jcb.200407073
Galectin-4 and sulfatides in apical membrane trafficking in enterocyte-like cells
Delphine Delacour et al. J Cell Biol. 2005.
Abstract
We have previously reported that 1-benzyl-2-acetamido-2-deoxy-alpha-D-galactopyranoside (GalNAc alpha-O-bn), an inhibitor of glycosylation, perturbed apical biosynthetic trafficking in polarized HT-29 cells suggesting an involvement of a lectin-based mechanism. Here, we have identified galectin-4 as one of the major components of detergent-resistant membranes (DRMs) isolated from HT-29 5M12 cells. Galectin-4 was also found in post-Golgi carrier vesicles. The functional role of galectin-4 in polarized trafficking in HT-29 5M12 cells was studied by using a retrovirus-mediated RNA interference. In galectin-4-depleted HT-29 5M12 cells apical membrane markers accumulated intracellularly. In contrast, basolateral membrane markers were not affected. Moreover, galectin-4 depletion altered the DRM association characteristics of apical proteins. Sulfatides with long chain-hydroxylated fatty acids, which were also enriched in DRMs, were identified as high-affinity ligands for galectin-4. Together, our data propose that interaction between galectin-4 and sulfatides plays a functional role in the clustering of lipid rafts for apical delivery.
Figures
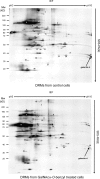
Figure 1.
Analysis of proteins contained in DRMs of control and GalNAcα-_O_-bn–treated HT-29 cells. 2-D patterns were obtained using 300 μg of DRM proteins isolated from control and GalNAcα-_O_-bn–treated (14 d) cells. Each protein spot was numbered and submitted to mass spectrometry analysis in MALDI-TOF mode. Spot number 32 was identified as galectin-4 (arrows).
Figure 2.
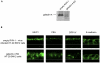
GalNAcα- O -bn decreases the apical localization of galectin-4. (A) Western blot of culture media, cytosol and membrane fractions of control and GalNAcα-_O_-bn–treated (14 d) cells after permeabilization with saponin, using anti–galectin-4 antibody. (B) Confocal microscopy with an anti–galectin-4 antibody on permeabilized or unpermeabilized control and GalNAcα-_O_-bn–treated (14 d) cells. Apical and xz sections are shown. Bars, 22 μm.
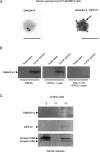
Figure 3.
Galectin-4 is associated with DRMs of post-Golgi carrier vesicles. (A) Immunogold labeling of nascent carrier vesicles isolated from HT-29 5M12 cells. Galectin-4 (arrowhead) was labeled with 18-nm gold particles and DPP-IV (arrow) by 12-nm gold. Bars, 156 nm. In the vesicle preparation, 30% of the vesicles were labeled with the anti–DPP-IV antibody. 20% of these DPP-IV–positive vesicles were also labeled with galectin-4. (B) Western blotting of galectin-4 in carrier vesicles. Trypsin digestion of galectin-4 in untreated or Triton X-100–treated carrier vesicles is shown. (C) Detergent extractability of galectin-4, DPP-IV, and annexins XIIIb and XIIIa in carrier vesicles from HT-29 5M12 cells. Detergent extracts, i.e., Triton X-100 soluble (S), insoluble at 4°C but soluble at 37°C (P1), and insoluble at 37°C (P2), were analyzed by Western blotting.
Figure 4.
Galectin-4 is no longer bound to sulfatides under GalNAcα- O -bn treatment. (A) Co-immunoprecipitation of galectin-4 complexes and HPTLC analysis of glycolipid ligands. Co-immunoprecipitation was performed from the same quantity of control and GalNAcα-_O_-bn–treated (14 d and 18 h) cells. Plates were iodine stained and scanned. The material migrating as two bands of galactosylceramides (Cer and Cer*) and the trailing corresponding to sulfatides (Sulf and Sulf*) were recovered from the HPTLC plate, submitted to acid-catalyzed methanolysis and analyzed by GC-MS. Cer, sphinganine and nonhydroxylated fatty acids; Cer*, sphinganine and 2-hydroxylated fatty acids; Sulf and Sulf*, sphingosine, sphinganine, phytosphingosine and 6-hydroxy-sphingosine, and 2-hydroxylated fatty acids from 16 to 28 carbon atoms. (B) Overlay experiments on the four fractions corresponding to the galactosylceramides and sulfatides purified from human sciatic nerve. Fractions were identified by their eluting methanol percent. Their glycosphingolipids were visualized by orcinol staining and analyzed for binding to the NH2-terminal CRD and the COOH-terminal CRD of galectin-4. Control without lectin is presented. arrows show the glycolipids which bind the NH2-terminal or COOH-terminal domain of galectin-4.
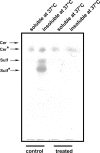
Figure 5.
Sulfatides are found in DRMs which are detergent insoluble at 37°C DRMs were isolated from a total membrane fraction of control and GalNAcα-_O_-bn–treated (14 d) cells. DRMs were further warmed at 37°C and both the soluble and insoluble material were collected and examined by HPTLC.
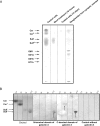
Figure 6.
KD of galectin-4 expression in HT-29 5M12 cells induces mistargeting of apical proteins. (A) Western blot analysis of galectin-4 in empty-RVH-1-virus–infected cells or galectin-4-KD cells. (B) Confocal microscopy with antibodies directed against apical (MUC1, CEA, DPP-IV) and basolateral (E-cadherin) proteins, on empty-RVH-1-virus–infected cells or galectin-4 KD cells. xz sections are shown.
Figure 7.
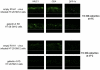
Apical glycoproteins are no longer associated with DRMs in galectin-4-KD HT-29 5M12 cells. Confocal microscopy with antibodies directed against MUC1, CEA, and DPP-IV, on empty-RVH-1-virus–infected cells or galectin-4-KD cells, after cell treatment with Triton X-100 at 4°C or 37°C. xz sections were shown.
Figure 8.
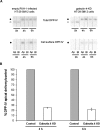
Apical and basolateral delivery of DPP-IV in control and galectin-4-KD cells. (A) Cells were pulse labeled for 30 min, and newly synthesized proteins were chased for 4 or 6 h and biotinylated from the apical or basolateral side. Aliquots from the immunoprecipitations (total) and streptavidin precipitations (cell surface) are shown. Ap, apical; Bl, basolateral. ○, DDP-IV precursor; •, mature DPP-IV. (B) Apical and total DPP-IV signals were quantitated in control and in galectin-4-KD cells. The apical to total ratio in control cells was set to 100% and apical transport efficiency in galectin-4-KD cells is shown relative to this value. Data are means ± SD.
Similar articles
- Galectin-4-regulated delivery of glycoproteins to the brush border membrane of enterocyte-like cells.
Stechly L, Morelle W, Dessein AF, André S, Grard G, Trinel D, Dejonghe MJ, Leteurtre E, Drobecq H, Trugnan G, Gabius HJ, Huet G. Stechly L, et al. Traffic. 2009 Apr;10(4):438-50. doi: 10.1111/j.1600-0854.2009.00882.x. Epub 2009 Jan 24. Traffic. 2009. PMID: 19192249 - 1-benzyl-2-acetamido-2-deoxy-alpha-D-galactopyranoside blocks the apical biosynthetic pathway in polarized HT-29 cells.
Delacour D, Gouyer V, Leteurtre E, Ait-Slimane T, Drobecq H, Lenoir C, Moreau-Hannedouche O, Trugnan G, Huet G. Delacour D, et al. J Biol Chem. 2003 Sep 26;278(39):37799-809. doi: 10.1074/jbc.M305755200. Epub 2003 Jul 10. J Biol Chem. 2003. PMID: 12855686 - Lipid raft organization and function in brush borders of epithelial cells.
Danielsen EM, Hansen GH. Danielsen EM, et al. Mol Membr Biol. 2006 Jan-Feb;23(1):71-9. doi: 10.1080/09687860500445604. Mol Membr Biol. 2006. PMID: 16611582 Review. - Galectin-2 at the enterocyte brush border of the small intestine.
Thomsen MK, Hansen GH, Danielsen EM. Thomsen MK, et al. Mol Membr Biol. 2009 Aug;26(5):347-55. doi: 10.1080/09687680903167781. Epub 2009 Aug 5. Mol Membr Biol. 2009. PMID: 19657968 - Recycling of galectin-3 in epithelial cells.
Hönig E, Schneider K, Jacob R. Hönig E, et al. Eur J Cell Biol. 2015 Jul-Sep;94(7-9):309-15. doi: 10.1016/j.ejcb.2015.05.004. Epub 2015 Jun 1. Eur J Cell Biol. 2015. PMID: 26059399 Review.
Cited by
- Using a Targeted Proteomics Chip to Explore Pathophysiological Pathways for Incident Diabetes- The Malmö Preventive Project.
Molvin J, Pareek M, Jujic A, Melander O, Råstam L, Lindblad U, Daka B, Leósdóttir M, Nilsson PM, Olsen MH, Magnusson M. Molvin J, et al. Sci Rep. 2019 Jan 22;9(1):272. doi: 10.1038/s41598-018-36512-y. Sci Rep. 2019. PMID: 30670722 Free PMC article. - From glycophenotyping by (plant) lectin histochemistry to defining functionality of glycans by pairing with endogenous lectins.
Kaltner H, García Caballero G, Ludwig AK, Manning JC, Gabius HJ. Kaltner H, et al. Histochem Cell Biol. 2018 Jun;149(6):547-568. doi: 10.1007/s00418-018-1676-7. Epub 2018 May 5. Histochem Cell Biol. 2018. PMID: 29730795 Review. - Probing sulfatide-tissue lectin recognition with functionalized glycodendrimersomes.
Murphy PV, Romero A, Xiao Q, Ludwig AK, Jogula S, Shilova NV, Singh T, Gabba A, Javed B, Zhang D, Medrano FJ, Kaltner H, Kopitz J, Bovin NV, Wu AM, Klein ML, Percec V, Gabius HJ. Murphy PV, et al. iScience. 2020 Dec 10;24(1):101919. doi: 10.1016/j.isci.2020.101919. eCollection 2021 Jan 22. iScience. 2020. PMID: 33409472 Free PMC article. - Sialylation of N-linked glycans mediates apical delivery of endolyn in MDCK cells via a galectin-9-dependent mechanism.
Mo D, Costa SA, Ihrke G, Youker RT, Pastor-Soler N, Hughey RP, Weisz OA. Mo D, et al. Mol Biol Cell. 2012 Sep;23(18):3636-46. doi: 10.1091/mbc.E12-04-0329. Epub 2012 Aug 1. Mol Biol Cell. 2012. PMID: 22855528 Free PMC article. - Galectin-9 trafficking regulates apical-basal polarity in Madin-Darby canine kidney epithelial cells.
Mishra R, Grzybek M, Niki T, Hirashima M, Simons K. Mishra R, et al. Proc Natl Acad Sci U S A. 2010 Oct 12;107(41):17633-8. doi: 10.1073/pnas.1012424107. Epub 2010 Sep 22. Proc Natl Acad Sci U S A. 2010. PMID: 20861448 Free PMC article.
References
- Alfalah, M., R. Jacob, U. Preuss, K.P. Zimmer, H. Naim, and H.Y. Naim. 1999. O-linked glycans mediate apical sorting of human intestinal sucrase-isomaltase through association with lipid rafts. Curr. Biol. 9:593–596. - PubMed
- Alfalah, M., R. Jacob, and H.Y. Naim. 2002. Intestinal dipeptidyl peptidase IV is efficiently sorted to the apical membrane through the concerted action of N- and O-glycans as well as association with lipid microdomains. J. Biol. Chem. 277:10683–10690. - PubMed
- Braccia, A., M. Villani, L. Immerdal, L.L. Niels-Christiansen, B.T. Nystrom, G. Hansen, and E.M. Danielsen. 2003. Microvillar membrane microdomains exist at physiological temperature. Role of galectin-4 as lipid raft stabilizer revealed by “superrafts”. J. Biol. Chem. 278:15679–15684. - PubMed
- Brown, D.A., and J.K. Rose. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 68:533–544. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources