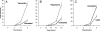Subversion of cellular autophagosomal machinery by RNA viruses - PubMed (original) (raw)
Subversion of cellular autophagosomal machinery by RNA viruses
William T Jackson et al. PLoS Biol. 2005 May.
Abstract
Infection of human cells with poliovirus induces the proliferation of double-membraned cytoplasmic vesicles whose surfaces are used as the sites of viral RNA replication and whose origin is unknown. Here, we show that several hallmarks of cellular autophagosomes can be identified in poliovirus-induced vesicles, including colocalization of LAMP1 and LC3, the human homolog of Saccharomyces cerevisiae Atg8p, and staining with the fluorophore monodansylcadaverine followed by fixation. Colocalization of LC3 and LAMP1 was observed early in the poliovirus replicative cycle, in cells infected with rhinoviruses 2 and 14, and in cells that express poliovirus proteins 2BC and 3A, known to be sufficient to induce double-membraned vesicles. Stimulation of autophagy increased poliovirus yield, and inhibition of the autophagosomal pathway by 3-methyladenine or by RNA interference against mRNAs that encode two different proteins known to be required for autophagy decreased poliovirus yield. We propose that, for poliovirus and rhinovirus, components of the cellular machinery of autophagosome formation are subverted to promote viral replication. Although autophagy can serve in the innate immune response to microorganisms, our findings are inconsistent with a role for the induced autophagosome-like structures in clearance of poliovirus. Instead, we argue that these double-membraned structures provide membranous supports for viral RNA replication complexes, possibly enabling the nonlytic release of cytoplasmic contents, including progeny virions, from infected cells.
Figures
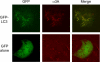
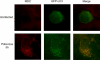
Figure 1. Simultaneous Visualization of GFP-LC3 and Poliovirus Protein 3A (Red)
MCF-7 cells were transfected either with a plasmid that expresses a GFP-LC3 fusion protein or GFP alone as indicated. Forty-eight hours posttransfection, cells were infected with poliovirus, then fixed and stained using a monoclonal antibody to poliovirus 3A protein and a rhodamine-conjugated secondary antibody.
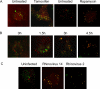
Figure 2. Simultaneous Visualization of GFP-LC3 and Resident Lysosomal Protein LAMP1 (Red) during Autophagic Induction, Poliovirus Infection, and Rhinovirus 14 Infection
(A) MCF-7 cells were transfected with a plasmid that expresses GFP-LC3 fusion protein and treated with 10 μM tamoxifen in DMSO/EtOH or with DMSO/EtOH alone as indicated. H1-HeLa cells were transfected with the GFP-LC3-expressing plasmid and treated with rapamycin for 5 h or left untreated as indicated. (B) A time course of poliovirus infection was performed in GFP-LC3-transfected MCF-7 cells, followed by visualization of LAMP1 and GFP-LC3 localization. Infections were with Mahoney type 1 poliovirus at a multiplicity of infection (MOI) of 50 PFU/cell for 5 h at 37 °C. (C) H1 HeLa cells were transfected with an LC3-GFP-expressing plasmid and either mock-infected or infected with human rhinoviruses as indicated at 50 infectious units/cell for 6 h at 33.5 °C.
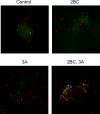
Figure 3. Simultaneous Visualization of GFP-LC3 and Resident Lysosomal Protein LAMP1 (Red) in Cells that Express Poliovirus 2BC and 3A Proteins
293T cells were transfected with vectors expressing 2BC, 3A, or both and a GFP-LC3 expressing vector for 48 h at 37 °C. Nonexpressing vector DNA was used to ensure that all transfections contained the same amount of DNA.
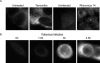
Figure 4. MDC Staining of MCF7 Cells upon Tamoxifen Treatment, Rhinovirus 14 Infection, or Poliovirus Infection
(A) Cells that were treated with 10 μM tamoxifen in DMSO/EtOH or with DMSO/EtOH alone for 48 h at 37 °C, or that were mock-infected or infected with human rhinovirus 14 as in Figure 3, were incubated with 100 μM MDC, fixed, and visualized by deconvolution microscopy in the UV channel as described in Materials and Methods. (B) Cells were infected with poliovirus at an MOI of 50 PFU/cell for 0, 1.5, 3, or 4.5 h at 37 °C; for each time point, MDC incubation was begun 1 h prior to fixation and visualization.
Figure 5. Simultaneous Visualization of GFP-LC3 Localization and MDC Staining (Red) in Uninfected and Poliovirus-Infected Cells
Forty-eight hours posttransfection of MCF-7 cells with a plasmid that expresses a GFP-LC3 fusion protein, cells were mock-infected or infected with poliovirus at an MOI of 50 PFU/cell for 5 h at 37 °C. After fixation, deconvolution microscopy was used to visualize fluorescence from both the MDC and GFP fluorescent molecules. MDC incubation was begun 1 h prior to fixation and visualization.
Figure 6. Intracellular Viral Yields from Poliovirus Infections Performed in the Presence of Pharmacological Inducers and Inhibitors of Autophagy
(A) H1 HeLa cells were treated with 10 μM tamoxifen in DMSO/EtOH or in DMSO/EtOH alone for 48 h at 37 °C. Cell numbers were determined, and triplicate plates were infected with poliovirus at an MOI of 0.1 PFU/cell for the times indicated. (B) H1 HeLa cells were treated with 50 nM rapamycin in DMSO/EtOH or DMSO/EtOH alone for 3 h before infection with poliovirus as in (A). (C) H1 HeLa cells were treated with 10 mM 3-methyladenine in DMSO/EtOH or DMSO/EtOH alone for 3 h before infection with poliovirus as in (A). Viral yields were determined by plaque assay in H1-HeLa cells and expressed as PFU/cell.
Figure 7. Intracellular and Extracellular Viral Yields from Poliovirus Infections of Cells Treated with Small RNA Duplexes to Reduce the Intracellular Concentrations of LC3 and Atg12p Proteins
(A) H1 HeLa cells (1 × 106) were transfected with 12.5 pm each of eight different RNA duplexes targeted to the LC3A and LC3B mRNAs, or with 100 pm of an RNA duplex targeted to firefly luciferase for 24 h at 37 °C (see Materials and Methods). Cell numbers were determined and triplicate plates were infected with poliovirus at an MOI of 0.1 PFU/cell for the times indicated. Viral yields were determined by plaque assay in H1-HeLa cells and expressed as PFU/cell for the intracellular virus, and as total PFU/plate for the extracellular virus. The relative abundance of LC3 protein in the cells incubated with the control and LC3-targeted RNAi molecules was determined by immunoblot using polyclonal antibodies directed against LC3 and GAPDH. (B) H1 HeLa cells were transfected with 25 pm each of four RNA duplexes targeted to ATG12 mRNA, or with 100 pm of an RNA duplex targeted to firefly luciferase, for 24 h at 37 °C (see Materials and Methods). Poliovirus infections were performed as in (A). The relative abundance of Atg12p protein was determined by immunoblot using antibodies against human Atg12p and GAPDH.
Figure 8. Ultrastructure of H1-HeLa Cells Infected with Human Rhinovirus 14 (HRV14) and Immunoelectron Microscopy of Cells Infected with Poliovirus
(A) Cells were infected with rhinovirus 14 as in Figure 3 and prepared for electron microscopy by high-pressure freezing. Examples of readily discernable double lipid bilayers are designated with large arrowheads; vesicles that contain intralumenal viral particles are denoted with small arrows. (B and C) Cells were transfected with a plasmid that expresses GFP-LC3 and subsequently infected with poliovirus for 5 h as in Figure 2. GFP-LC3 was visualized using a secondary antibody coupled to 10-nm gold particles; examples of such particles are denoted with arrows. An arrowhead identifies apparently extracellular packets of cytosol. (D) Cells transfected with an GFP-LC3-expressing plasmid and infected with poliovirus as in (C) were immunostained using an antibody directed against VP1, a viral capsid protein and visualized using a secondary antibody conjugated to 10-nm gold particles; examples of such particles are identified with arrows.
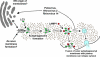
Figure 9. Pathway of Autophagosome Formation, Autophagic Degradation, and Proposed Steps of Pathway Subversion by Poliovirus and Related Viruses
Double-membraned autophagosomes form either from ER membrane or de novo, encapsulating cytosol; the action of many gene products, including Atg5p and Atg12p, are required. LC3 protein (the Atg8p homolog) is associated with “sequestration crescents” as well as fully formed double-membraned autophagosomes. LAMP1 acquisition is a hallmark of the maturation of these structures, which eventually fuse with lysosomes to produce mature autophagosomes with single membranes and electron-dense contents. We hypothesize that infection by poliovirus or rhinovirus induces accumulation of autophagosomes to promote viral RNA replication by accelerating the formation of autophagosome-like structures from ER membranes, blocking the maturation of these structures into degradative organelles, or both (upper dotted line). The double-membraned topology makes the extracellular release of virions trapped in the cytosolic lumen topologically plausible, providing a mechanism for viral release in the absence of cell lysis. This could occur either from a double-membraned structure or from one in which only one of the membranes remained (dotted arrows).
Similar articles
- Potential subversion of autophagosomal pathway by picornaviruses.
Taylor MP, Kirkegaard K. Taylor MP, et al. Autophagy. 2008 Apr;4(3):286-9. doi: 10.4161/auto.5377. Epub 2007 Dec 5. Autophagy. 2008. PMID: 18094610 Review. - Topology of double-membraned vesicles and the opportunity for non-lytic release of cytoplasm.
Kirkegaard K, Jackson WT. Kirkegaard K, et al. Autophagy. 2005 Oct-Dec;1(3):182-4. doi: 10.4161/auto.1.3.2065. Epub 2005 Oct 30. Autophagy. 2005. PMID: 16874042 - Role of microtubules in extracellular release of poliovirus.
Taylor MP, Burgon TB, Kirkegaard K, Jackson WT. Taylor MP, et al. J Virol. 2009 Jul;83(13):6599-609. doi: 10.1128/JVI.01819-08. Epub 2009 Apr 15. J Virol. 2009. PMID: 19369338 Free PMC article. - Modification of cellular autophagy protein LC3 by poliovirus.
Taylor MP, Kirkegaard K. Taylor MP, et al. J Virol. 2007 Nov;81(22):12543-53. doi: 10.1128/JVI.00755-07. Epub 2007 Sep 5. J Virol. 2007. PMID: 17804493 Free PMC article. - Interplay between the cellular autophagy machinery and positive-stranded RNA viruses.
Shi J, Luo H. Shi J, et al. Acta Biochim Biophys Sin (Shanghai). 2012 May;44(5):375-84. doi: 10.1093/abbs/gms010. Epub 2012 Feb 16. Acta Biochim Biophys Sin (Shanghai). 2012. PMID: 22343377 Free PMC article. Review.
Cited by
- Macrophage autophagy in immunity to Cryptococcus neoformans and Candida albicans.
Nicola AM, Albuquerque P, Martinez LR, Dal-Rosso RA, Saylor C, De Jesus M, Nosanchuk JD, Casadevall A. Nicola AM, et al. Infect Immun. 2012 Sep;80(9):3065-76. doi: 10.1128/IAI.00358-12. Epub 2012 Jun 18. Infect Immun. 2012. PMID: 22710871 Free PMC article. - Swine acute diarrhea syndrome coronavirus induces autophagy to promote its replication via the Akt/mTOR pathway.
Zeng S, Zhao Y, Peng O, Xia Y, Xu Q, Li H, Xue C, Cao Y, Zhang H. Zeng S, et al. iScience. 2022 Nov 18;25(11):105394. doi: 10.1016/j.isci.2022.105394. Epub 2022 Oct 20. iScience. 2022. PMID: 36281226 Free PMC article. - Identification of novel human damage response proteins targeted through yeast orthology.
Svensson JP, Fry RC, Wang E, Somoza LA, Samson LD. Svensson JP, et al. PLoS One. 2012;7(5):e37368. doi: 10.1371/journal.pone.0037368. Epub 2012 May 16. PLoS One. 2012. PMID: 22615993 Free PMC article. - PAMPs and DAMPs: signal 0s that spur autophagy and immunity.
Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. Tang D, et al. Immunol Rev. 2012 Sep;249(1):158-75. doi: 10.1111/j.1600-065X.2012.01146.x. Immunol Rev. 2012. PMID: 22889221 Free PMC article. Review. - Dysregulation of autophagy in murine fibroblasts resistant to HSV-1 infection.
Le Sage V, Banfield BW. Le Sage V, et al. PLoS One. 2012;7(8):e42636. doi: 10.1371/journal.pone.0042636. Epub 2012 Aug 10. PLoS One. 2012. PMID: 22900036 Free PMC article.
References
- Dales S, Eggers HJ, Tamm I, Palade GE. Electron microscopic study of the formation of poliovirus. Virology. 1965;26:379–389. - PubMed
- Bienz K, Egger D, Pasamontes L. Association of polioviral proteins of the P2 genomic region with the viral replication complex and virus-induced membrane synthesis as visualized by electron microscopic immunocytochemistry and autoradiography. Virology. 1987;60:220–226. - PubMed
- Cho MW, Teterina N, Egger D, Bienz K, Ehrenfeld E. Membrane rearrangement and vesicle induction by recombinant poliovirus 2C and 2BC in human cells. Virology. 1994;202:129–145. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous