A three-stemmed mRNA pseudoknot in the SARS coronavirus frameshift signal - PubMed (original) (raw)
A three-stemmed mRNA pseudoknot in the SARS coronavirus frameshift signal
Ewan P Plant et al. PLoS Biol. 2005 Jun.
Abstract
A wide range of RNA viruses use programmed -1 ribosomal frameshifting for the production of viral fusion proteins. Inspection of the overlap regions between ORF1a and ORF1b of the SARS-CoV genome revealed that, similar to all coronaviruses, a programmed -1 ribosomal frameshift could be used by the virus to produce a fusion protein. Computational analyses of the frameshift signal predicted the presence of an mRNA pseudoknot containing three double-stranded RNA stem structures rather than two. Phylogenetic analyses showed the conservation of potential three-stemmed pseudoknots in the frameshift signals of all other coronaviruses in the GenBank database. Though the presence of the three-stemmed structure is supported by nuclease mapping and two-dimensional nuclear magnetic resonance studies, our findings suggest that interactions between the stem structures may result in local distortions in the A-form RNA. These distortions are particularly evident in the vicinity of predicted A-bulges in stems 2 and 3. In vitro and in vivo frameshifting assays showed that the SARS-CoV frameshift signal is functionally similar to other viral frameshift signals: it promotes efficient frameshifting in all of the standard assay systems, and it is sensitive to a drug and a genetic mutation that are known to affect frameshifting efficiency of a yeast virus. Mutagenesis studies reveal that both the specific sequences and structures of stems 2 and 3 are important for efficient frameshifting. We have identified a new RNA structural motif that is capable of promoting efficient programmed ribosomal frameshifting. The high degree of conservation of three-stemmed mRNA pseudoknot structures among the coronaviruses suggests that this presents a novel target for antiviral therapeutics.
Figures
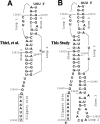
Figure 1. Different Representations of the SARS-CoV Frameshift Signal
(A) Two-stemmed H-type mRNA pseudoknot proposed by Thiel et al. [8]. (B) Three-stemmed mRNA pseudoknot structure investigated in this study.
Figure 2. Multiple Sequence Alignment of the SARS-CoV −1 PRF with Nine Homologous Signals Found in Other Coronavirus Genomes
AIBV, avian infectious bronchitis virus; BCoV, bovine coronavirus; HCoV-229E, human coronavirus 229E; HCoV-HKU1; HCoV-NL63, human coronavirus NL63; HCoV-OC43, human coronavirus OC43; MHV, murine hepatitis virus; PEDV, porcine epidemic diarrhea virus; SARS, SARS coronavirus; TGV, transmissible gastroenteritis virus. Heptameric slippery sites are indicated in brown; dashes indicate gaps in the sequence alignments; basepairing positions involved in the consensus first, second, and third helices are denoted by blue, red, and green nucleotides, respectively. Downstream regions homologous to the kissing loop known to promote frameshifting in HCoV-229E [16,17]. HCoV-229R, HCoV-NL, PEDV, and TGV are also highlighted in red with the flanking stem-forming sequences underlined. Asterisks indicate perfectly conserved positions in primary sequence.
Figure 3. Phylogenetic Analyses of Coronavirus −1 PRF Signals
Unrooted tree constructed based on the multiple sequence alignment from Figure 2.
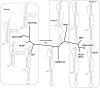
Figure 4. Secondary Structure Mapping of the SARS-CoV Frameshift Signal
(A and B) The results of nuclease cleavage of RNA from nucleotides 13400–13470 of SARS-CoV. RNAs were 5′ end labeled with 32P and subjected to enzymatic digestion, as described in Materials and Methods. The three different concentrations of each nuclease are indicated by the triangles are described in Materials and Methods. C denotes undigested control, and OH− denotes hydrolysis ladders. (C) Interpretation of nuclease digestion analyses mapped onto the proposed secondary structure of the SARS-CoV frameshift signal. Nuclease cleavage sites, proposed basepairs, and specific bases protected from nuclease attack are indicated.
Figure 5. NMR Data Were Collected at 25 °C at a Proton Resonance Frequency of 900 MHz
(A) Secondary structure of the SARS-CoV frameshift pseudoknot (residues 13405–13472). Different color coding was used to denote basepaired regions in stems 1 (cyan), 2 (green), and 3 (grey and blue). Only the last two digits of the wild-type sequence numbering are used for clarity. (B) Imino region of a one-dimensional jump-return echo spectrum of SARS-CoV pseudoknot. (C) Portion of a 2D 1H,1H-NOESY. Sequential imino-imino proton NOE assignment paths are shown by different colors for stem 1 (cyan), stem 2 (green), and stem 3 (black and blue). (D) 2D Quantitative J(N,N) HNN-COSY spectrum showing interstrand 1H3–15N3(U) to 15N1(A) and 1H1–15N1(G) to 15N3(C) correlations. Data were collected on a uniformly 13C/15N-labeled sample. Red peaks correspond to diagonal resonances and are labeled with assignment information for the basepaired stem regions matching the color coding in (C). Green cross peaks are caused by scalar cross hydrogen bond 2h J(N,N) couplings detected using a defocusing delay of 36 ms. Carrier positions were on water for 1H and 185 ppm.
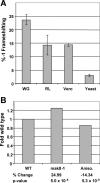
Figure 6. Functional Characterization of the SARS-CoV Frameshift Signal
(A) The wild-type SARS-CoV frameshift signal promotes efficient frameshifting in vitro and in vivo. Programmed −1 ribosomal frameshifting was monitored in wheat germ and rabbit reticulocyte lysates in vitro, and in Vero epithelial cells and yeast in vivo, as described in Materials and Methods. Error bars denote the standard error. (B) SARS-CoV-directed −1 PRF was monitored in wild-type yeast cells with or without anisomycin (20 μg/ml), or in isogenic RPL3 gene deletion cells expressing either the wild-type or mak8–1 alleles of RPL3 on an episomal plasmid [21]. Changes in −1 PRF efficiencies are shown as fold wild-type, and _p_-values are shown as described previously [25].
Figure 7. Molecular Genetic Analyses of Stems 1 and 3
Constructs used to examine the contributions of stem structures and bulged adenosine residues to programmed −1 ribosomal frameshifting are depicted. Shading is used to indicate mutagenized bases. Programmed −1 ribosomal frameshifting promoted by the wild-type SARS-CoV −1 PRF signal was monitored in Vero, as described in the Materials and Methods. Standard deviations (S.D.) are indicated for each sample, as previously described [25]. The S2 series (above) examines the roles of structures and bases in stem 2. The S3 series (below) examines the roles of structures and bases in stem 3.
Similar articles
- An atypical RNA pseudoknot stimulator and an upstream attenuation signal for -1 ribosomal frameshifting of SARS coronavirus.
Su MC, Chang CT, Chu CH, Tsai CH, Chang KY. Su MC, et al. Nucleic Acids Res. 2005 Jul 29;33(13):4265-75. doi: 10.1093/nar/gki731. Print 2005. Nucleic Acids Res. 2005. PMID: 16055920 Free PMC article. - Achieving a golden mean: mechanisms by which coronaviruses ensure synthesis of the correct stoichiometric ratios of viral proteins.
Plant EP, Rakauskaite R, Taylor DR, Dinman JD. Plant EP, et al. J Virol. 2010 May;84(9):4330-40. doi: 10.1128/JVI.02480-09. Epub 2010 Feb 17. J Virol. 2010. PMID: 20164235 Free PMC article. - Programmed ribosomal frameshifting in HIV-1 and the SARS-CoV.
Brierley I, Dos Ramos FJ. Brierley I, et al. Virus Res. 2006 Jul;119(1):29-42. doi: 10.1016/j.virusres.2005.10.008. Epub 2005 Nov 28. Virus Res. 2006. PMID: 16310880 Free PMC article. Review. - The role of programmed-1 ribosomal frameshifting in coronavirus propagation.
Plant EP, Dinman JD. Plant EP, et al. Front Biosci. 2008 May 1;13:4873-81. doi: 10.2741/3046. Front Biosci. 2008. PMID: 18508552 Free PMC article. Review.
Cited by
- Discovery and Quantification of Long-Range RNA Base Pairs in Coronavirus Genomes with SEARCH-MaP and SEISMIC-RNA.
Allan MF, Aruda J, Plung JS, Grote SL, des Taillades YJM, de Lajarte AA, Bathe M, Rouskin S. Allan MF, et al. Res Sq [Preprint]. 2024 Aug 7:rs.3.rs-4814547. doi: 10.21203/rs.3.rs-4814547/v1. Res Sq. 2024. PMID: 39149495 Free PMC article. Preprint. - Rational design of a synthetic mammalian riboswitch as a ligand-responsive -1 ribosomal frame-shifting stimulator.
Lin YH, Chang KY. Lin YH, et al. Nucleic Acids Res. 2016 Oct 14;44(18):9005-9015. doi: 10.1093/nar/gkw718. Epub 2016 Aug 12. Nucleic Acids Res. 2016. PMID: 27521370 Free PMC article. - An intricate balancing act: Upstream and downstream frameshift co-regulatory elements.
Lee S, Yan S, Dey A, Laederach A, Schlick T. Lee S, et al. bioRxiv [Preprint]. 2024 Jun 27:2024.06.27.599960. doi: 10.1101/2024.06.27.599960. bioRxiv. 2024. PMID: 38979256 Free PMC article. Preprint. - Two Years into the COVID-19 Pandemic: Lessons Learned.
da Silva SJR, do Nascimento JCF, Germano Mendes RP, Guarines KM, Targino Alves da Silva C, da Silva PG, de Magalhães JJF, Vigar JRJ, Silva-Júnior A, Kohl A, Pardee K, Pena L. da Silva SJR, et al. ACS Infect Dis. 2022 Sep 9;8(9):1758-1814. doi: 10.1021/acsinfecdis.2c00204. Epub 2022 Aug 8. ACS Infect Dis. 2022. PMID: 35940589 Free PMC article. Review. - Sequence analysis of SARS-CoV-2 genome reveals features important for vaccine design.
Kames J, Holcomb DD, Kimchi O, DiCuccio M, Hamasaki-Katagiri N, Wang T, Komar AA, Alexaki A, Kimchi-Sarfaty C. Kames J, et al. bioRxiv [Preprint]. 2020 Mar 31:2020.03.30.016832. doi: 10.1101/2020.03.30.016832. bioRxiv. 2020. PMID: 32511300 Free PMC article. Updated. Preprint.
References
- Marra MA, Jones SJ, Astell CR, Holt RA, Brooks-Wilson A, et al. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. - PubMed
- Rota PA, Oberste MS, Monroe SS, Nix WA, Campagnoli R, et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. - PubMed
- Brierley I. Ribosomal frameshifting on viral RNAs. J Gen Virol. 1995;76:1885–1892. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous