An animal model of SARS produced by infection of Macaca mulatta with SARS coronavirus - PubMed (original) (raw)
Jianwei Wang, Qiang Wei, Mingpeng She, Wayne A Marasco, Hong Jiang, Xinming Tu, Hua Zhu, Lili Ren, Hong Gao, Li Guo, Lan Huang, Renquan Yang, Zhe Cong, Lan Guo, Yanbin Wang, Yali Liu, Yili Sun, Shumin Duan, Jianguo Qu, Liangbiao Chen, Wei Tong, Li Ruan, Peimao Liu, Hua Zhang, Jianmin Zhang, Huiyuan Zhang, Depei Liu, Qian Liu, Tao Hong, Wei He
Affiliations
- PMID: 15892035
- PMCID: PMC7167940
- DOI: 10.1002/path.1769
An animal model of SARS produced by infection of Macaca mulatta with SARS coronavirus
Chuan Qin et al. J Pathol. 2005 Jul.
Abstract
A new SARS animal model was established by inoculating SARS coronavirus (SARS-CoV) into rhesus macaques (Macaca mulatta) through the nasal cavity. Pathological pulmonary changes were successively detected on days 5-60 after virus inoculation. All eight animals showed a transient fever 2-3 days after inoculation. Immunological, molecular biological, and pathological studies support the establishment of this SARS animal model. Firstly, SARS-CoV-specific IgGs were detected in the sera of macaques from 11 to 60 days after inoculation. Secondly, SARS-CoV RNA could be detected in pharyngeal swab samples using nested RT-PCR in all infected animals from 5 days after virus inoculation. Finally, histopathological changes of interstitial pneumonia were found in the lungs during the 60 days after viral inoculation: these changes were less marked at later time points, indicating that an active healing process together with resolution of an acute inflammatory response was taking place in these animals. This animal model should provide insight into the mechanisms of SARS-CoV-related pulmonary disease and greatly facilitate the development of vaccines and therapeutics against SARS.
Copyright 2005 Pathological Society of Great Britain and Ireland. Published by John Wiley & Sons, Ltd.
Figures
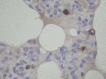
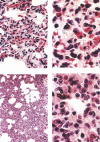
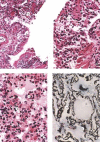
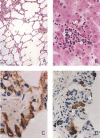
Figure 1
SARS‐CoV antigen in lung tissues from infected Macaca mulatta (No 1951) 10 days after infection. Lung tissue samples were stained using an avidin–biotin complex peroxidase technique. Sections were incubated with monoclonal antibodies against SARS‐CoV (1 : 400) and were developed using biotin‐labelled goat anti‐mouse antibody. (Immunohistochemical staining; original magnification ×400)
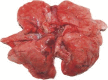
Figure 2
Macroscopic appearance of lung tissue of SARS‐CoV‐infected monkey 2373 on the fifth day after infection; local lesions are arrowed
Figure 3
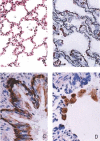
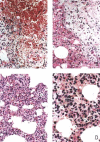
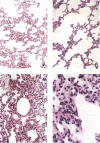
Lung alveoli of a normal healthy rhesus monkey for comparison. (A) Normal alveolar septa (H&E; original magnification ×200). (A, a) The inter‐alveolar septa are narrow; there is no inflammatory cell infiltration or oedema (H&E; original magnification ×200). (A, b) The alveolar sacs are clear; no inflammatory cells are seen in any of the alveoli shown (H&E; original magnification ×200). Panel B, a shows intact reticulin fibres in the alveolar septa; B, b shows small capillaries located in the inter‐alveolar septa (MCT; original magnification ×400). Panel C shows the intact lining epithelium; C, a, b shows positive staining of epithelial cells with a monoclonal anti‐cytokeratin antibody (CK +; original magnification ×1000). Panel D, a, b shows that foam cells staining positive for CD68 are adherent to regenerating epithelial cells (MAb against human CD68; original magnification ×1000)
Figure 4
Monkey 2373, 5 days after infection. (A, a) Interstitial pneumonia with haemorrhage; (A, b) foam cells (macrophages) with engulfed red blood cells in the cytoplasm (H&E; original magnification ×200). (B, a) Interstitial pneumonia: macrophage infiltration is remarkable, with engulfed red blood cells in their cytoplasm; (B, b) lymphocytes and fibrin deposition; (B, c) pleuritis with oedema, phagocyte and lymphocyte infiltration (H&E; original magnification ×200). (C, a) Haemorrhage; (C, b) macrophages; (C, c) lymphocytes and macrophages (H&E; original magnification ×200). (D, a) Macrophages packed in the alveolus; (D, b) lymphocytes; (D, c) foam cells (H&E; original magnification ×400)
Figure 5
Monkey 1924, 7 days after infection. (A, a) Haemorrhage in the inter‐alveolar septum; (A, b) engulfed red blood cells in phagocytes; (A, c) alveoli filled with fibrin, inflammatory cells, and red blood cells; (A, d) lung oedema (HE; original magnification ×400). (B, a) Macrophage with engulfed red blood cell; (B, b) foam cells; (B, c) lymphocytes (H&E; original magnification ×1000). (C, a) Interstitial pneumonia; (C, b) distended air sacs; (C, c) dilatation of respiratory duct (H&E; original magnification ×200). (D, a) Macrophages; (D, b) lymphocytes in the inter‐alveolar septum (H&E; original magnification ×1000)
Figure 6
Monkey 1951, 10 days after infection. (A, a) Granulation tissue in the damaged bronchiolar wall; (A, b) proliferation of the lining epithelial cells; (A, c) early regeneration of the damaged epithelium (H&E; original magnification ×200). (B, a) Phagocytes; (B, b) lymphocytes (H&E; original magnification ×400). (C, a) Lung oedema; (C, b) interstitial pneumonia; (C, c) foam cells (H&E; original magnification ×400). (D, a) Fragmentation of reticulin fibres in the alveolar wall; (D, b) desquamation of epithelial cells (Gomori's stain; ×1000)
Figure 7
Monkey 883, 15 days after infection. (A, a) Interstitial pneumonia with haemorrhage and fibrin deposition; (A, b) phagocytes with red blood cell engulfment; (A, c) capillary congestion (H&E; original magnification ×200). (B, a) Presence of fibrin filaments between neighbouring alveoli; (B, b) red blood cells engulfed in phagocytes; (B, c) foam cells (H&E; original magnification ×400). (C, a) Lung oedema; (C, b) accumulation of phagocytes in alveoli; (C, c) lymphocytes in the alveolar septum and the alveoli (H&E; original magnification ×400). (D, a) Interstitial pneumonia present in monkey 227
Figure 8
Monkey 1921, 30 days after infection. (A, a) Interstitial pneumonia with mononuclear cell infiltration; (A, b) localized emphysema (H&E; original magnification ×100). (B, a) Focal liver necrosis (No 1921); (B, b) degenerate and necrotic cells; (B, c) lymphocytes; (B, d) liver cells. (C, a) Regenerative epithelial cells shown to be CK‐positive (cytokeratin MAb stain; original magnification ×1000); (C, b) macrophages. (D, a) CD68‐positive cells (macrophages) adherent to the surface of regenerative epithelial cells (CD68 MAb stain; original magnification ×1000); (D, b) regenerative epithelial cells in small bronchioles
Figure 9
Monkeys 900 and 2372, 60 days after infection. (A, a) Interstitial pneumonia with mononuclear infiltration (H&E; original magnification ×50) (No 2372). (A, b) Some alveoli are distended. (B, a) Mild interstitial pneumonia; (B, b) emphysema (H&E; original magnification × 100) (No 900). (C, a) Interstitial pneumonia and infiltration of mononuclear cells; (C, b) foam cell formation (H&E; original magnification ×200). (D, a) Foam cells in alveoli; (D, b) phagocytes; (D, c) lymphocytes in septa (H&E; original magnification ×400)
Similar articles
- Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection.
Liu L, Wei Q, Lin Q, Fang J, Wang H, Kwok H, Tang H, Nishiura K, Peng J, Tan Z, Wu T, Cheung KW, Chan KH, Alvarez X, Qin C, Lackner A, Perlman S, Yuen KY, Chen Z. Liu L, et al. JCI Insight. 2019 Feb 21;4(4):e123158. doi: 10.1172/jci.insight.123158. eCollection 2019 Feb 21. JCI Insight. 2019. PMID: 30830861 Free PMC article. - Intratracheal inoculation of severe acute respiratory syndrome coronavirus in monkeys Macaca rhesus.
Luo F, Hou W, Yang ZQ, Tang ZJ, Wang Y, Xian QY, Sun LH. Luo F, et al. Acta Virol. 2007;51(3):171-7. Acta Virol. 2007. PMID: 18076307 - Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome.
Kuiken T, Fouchier RA, Schutten M, Rimmelzwaan GF, van Amerongen G, van Riel D, Laman JD, de Jong T, van Doornum G, Lim W, Ling AE, Chan PK, Tam JS, Zambon MC, Gopal R, Drosten C, van der Werf S, Escriou N, Manuguerra JC, Stöhr K, Peiris JS, Osterhaus AD. Kuiken T, et al. Lancet. 2003 Jul 26;362(9380):263-70. doi: 10.1016/S0140-6736(03)13967-0. Lancet. 2003. PMID: 12892955 Free PMC article. - SARS-CoV replication and pathogenesis in an in vitro model of the human conducting airway epithelium.
Sims AC, Burkett SE, Yount B, Pickles RJ. Sims AC, et al. Virus Res. 2008 Apr;133(1):33-44. doi: 10.1016/j.virusres.2007.03.013. Epub 2007 Apr 23. Virus Res. 2008. PMID: 17451829 Free PMC article. Review. - The role of epidermal growth factor receptor (EGFR) signaling in SARS coronavirus-induced pulmonary fibrosis.
Venkataraman T, Frieman MB. Venkataraman T, et al. Antiviral Res. 2017 Jul;143:142-150. doi: 10.1016/j.antiviral.2017.03.022. Epub 2017 Apr 5. Antiviral Res. 2017. PMID: 28390872 Free PMC article. Review.
Cited by
- The expression of membrane protein augments the specific responses induced by SARS-CoV nucleocapsid DNA immunization.
Shi SQ, Peng JP, Li YC, Qin C, Liang GD, Xu L, Yang Y, Wang JL, Sun QH. Shi SQ, et al. Mol Immunol. 2006 Apr;43(11):1791-8. doi: 10.1016/j.molimm.2005.11.005. Epub 2006 Jan 19. Mol Immunol. 2006. PMID: 16423399 Free PMC article. - A review of studies on animal reservoirs of the SARS coronavirus.
Shi Z, Hu Z. Shi Z, et al. Virus Res. 2008 Apr;133(1):74-87. doi: 10.1016/j.virusres.2007.03.012. Epub 2007 Apr 23. Virus Res. 2008. PMID: 17451830 Free PMC article. Review. - Should we be concerned about COVID-19 with nonhuman primates?
Santos WJ, Guiraldi LM, Lucheis SB. Santos WJ, et al. Am J Primatol. 2020 Aug;82(8):e23158. doi: 10.1002/ajp.23158. Epub 2020 Jun 4. Am J Primatol. 2020. PMID: 32495390 Free PMC article. - Infection of SARS-CoV on juvenile and adult Brandt's vole_Microtus brandtii_.
Gao H, Peng J, Deng W, Shi D, Bao L, Wang D, Zhang B, Qin C, Zhang Z. Gao H, et al. Chin Sci Bull. 2005;50(12):1199-1204. doi: 10.1007/BF03183693. Chin Sci Bull. 2005. PMID: 32214720 Free PMC article. - Spatiotemporal interplay of severe acute respiratory syndrome coronavirus and respiratory mucosal cells drives viral dissemination in rhesus macaques.
Liu L, Wei Q, Nishiura K, Peng J, Wang H, Midkiff C, Alvarez X, Qin C, Lackner A, Chen Z. Liu L, et al. Mucosal Immunol. 2016 Jul;9(4):1089-101. doi: 10.1038/mi.2015.127. Epub 2015 Dec 9. Mucosal Immunol. 2016. PMID: 26647718 Free PMC article.
References
- Marra MA, Jones SJ, Astell CR, et al. The genome sequence of the SARS‐associated coronavirus. Science 2003; 300: 1399–1404. - PubMed
- Drosten C, Gunther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 2003; 348: 1967–1976. - PubMed
- Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 2003; 348: 1953–1966. - PubMed
- Calza L, Manfredi R, Verucchi G, et al. SARS: a new emergency in the world health. Recent Prog Med 2003; 94: 284–294. - PubMed
- Chiu RW, Chim SS, Lo YM. Molecular epidemiology of SARS—from Amoy Gardens to Taiwan. N Engl J Med 2003; 349: 1875–1876. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous