Dendritic cells maximize the memory CD8 T cell response to infection - PubMed (original) (raw)
Dendritic cells maximize the memory CD8 T cell response to infection
David J Zammit et al. Immunity. 2005 May.
Abstract
Costimulatory signals from dendritic cells (DCs) are required for naive T cells to respond to antigenic stimulation. To what extent DCs reactivate memory T cells during recall responses is not known. Here, an in vivo depletion system has been used to analyze the role of DCs in reactivating CD8 memory T cells during recall responses to three different microbial infections. We show a profound decrease in the numbers of responding memory CD8 T cells in both lymphoid and nonlymphoid tissues during the recall responses to infection with vesicular stomatitis virus, Listeria monocytogenes (Lm), or influenza virus. These data show that interaction with DCs is a major mechanism driving T cell reactivation in vivo, even during a tissue-specific infection of the respiratory tract.
Figures
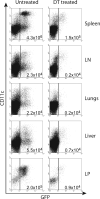
Figure 1. DCs Are Ablated in Lymphoid and Nonlymphoid Organs after DT Treatment
DTR BM chimeras were injected with two doses of either 4 ng/g body weight DT or PBS 3 days apart and 24 hr later analyzed to determine the efficacy of DC ablation. Pooled samples of liver, peripheral LN, and LP from the two groups of mice were used to gain enough numbers of DCs for analysis. DCs were identified as MHC II+ CD11c+ GFP+ by flow cytometry, and the plots shown are gated on MHC class II+ cells. DC numbers in all organs tested were generally depleted by ~90% after treatment. The total number of DCs is shown in each plot. Four mice were used per group and the experiment repeated twice. Data shown is representative of one experiment.
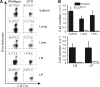
Figure 2. DCs Are Required for the Initiation of an Anti-Lm-Ova Recall Response
DTR or wild-type (wt) bone marrow-reconstituted mice were treated 4 and 1 days before infection with DT to ablate DCs in the DTR mice. Ly5.2+ ova-specific CD8+ memory cells were transferred into both groups 1 day before the mice were infected i.v. with 103 cfu Lm-ova. DT was administered every 3 days, and the mice were analyzed 6 days after infection for Ly5.2+ ova-specific CD8+ T cells. (A) The proportion of Ly5.2+ ova+ cells was decreased in all organs analyzed. The numbers in the upper left corner of each panel represent percent of ova tet+ LFA+ cells among the donor CD8+ T cells. (B) The total number of Ly5.2+ ova-specific cells is dramatically less in the absence of DCs in all organs and was calculated from the proportion of tet+ cells and total cellularity of each organ. The data represent a total of six mice per group from two individual experiments. All values are means ± SEM.
Figure 3. DCs Are Required for the Initiation of an Anti-VSV Recall Response
DTR or wt bone marrow-reconstituted mice were treated after the protocol in Figure 2 except the mice were infected i.v. with 105 pfu VSV. DT was administered every 3 days, and the mice were analyzed 6 days after infection for 5.2+ N tet+ CD8+ T cells. (A) The proportion of Ly5.2+ N tet+ cells is decreased in all organs analyzed to varying degrees. The numbers in the upper left corner of each panel represent percent of N tet+ LFA+ cells among the donor CD8+ T cells. (B) The total number of Ly5.2+ N tet+ cells was calculated from the proportion of tet+ cells and total cellularity of each organ. The data is from one experiment of three mice per group, and the values are means ± SEM. The experiment was repeated twice with similar results.
Figure 4. CD8 Memory T Cells Respond to Peptide-Loaded DCs in DC-Depleted Mice
VSV-specific CD8 memory T cells (50,000) were transferred to the indicated chimeras, and all were treated with DT on the day of transfer. One day later, 5 × 105 purified splenic DCs (either coated with the VSV N peptide or not) were transferred i.v. 6 days later, the response in the lung (shown here) and the spleen (data not shown) was measured by flow cytometry using an N peptide H-2Kb tetramer. Data shown is gated on CD8+ T cells. Responses in the spleen, though less robust, were similar between groups.
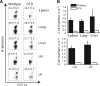
Figure 5. Partial DC Requirement for the Initiation of an Anti-Influenza Recall Response
DTR or wt bone marrow-reconstituted mice were treated following the protocol in Figure 2 except the mice were infected i.n. with 103 pfu WSN-ova. DT was administered every 3 days, and the mice were analyzed 8 days after infection for Ly5.2+ ova-specific CD8+ T cells. (A) The proportion of Ly5.2+ ova+ cells is decreased in all organs analyzed with the exception of the MedLN. The numbers in the upper left corner of each panel represent percent of ova tet+ LFA+ cells among the donor CD8+ T cells. (B) The total number of Ly5.2+ ova+ cells was calculated from the proportion of tet+ cells and total cellularity of each organ,. The data represents five mice (wt) or six mice (DTR) from two individual experiments. All values are means ± SEM.
Figure 6. The Reduced Recall Response to Influenza Is Delayed in the Absence of DCs
(A) The total cellularity of the lungs, BAL fluid, and spleen is equal in the DTR and wt mice, 11 days after infection. The cellularity of the MedLN is still dramatically reduced at this time point. (B) Despite the equal total cellularity in the BAL fluid and spleen, there is still a reduced number of ova+ CD8+ cells in these organs. By day 11, the number of ova+ CD8+ cells in the lungs of wt and DTR mice are comparable. The data represent six mice (wt) or four mice (DTR) from two individual experiments. All values are means ± SEM.
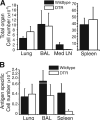
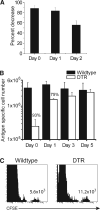
Figure 7. DCs Are Required for More than 48 hr to Generate a Full Recall Response
(A) Ly5.2+ ova -specific CD8+ memory cells were transferred into both groups of mice 1 day before the mice were infected i.v. with 105 pfu VSV-ova. A single dose of DT was administered at either day 0, 1, or 2 of infection and CD8+ Ly5.2+ ova-tet+ cells in the spleens were quantitated six days after infection. The percent decrease was calculated based on total tet+ cell numbers in each group. The data represent four mice in each group from two individual experiments. (B) CFSE-labeled Ly5.2+ VSV N protein-specific CD8+ memory cells were transferred into the indicated chimeras 1 day before the mice were infected i.v. with 105 pfu VSV. A single dose of DT was administered at either day 0, 1, 3, or 5, and CD8+ Ly5.2+ N tet+ cells in the spleens were quantitated six days after infection. Values represent total tet+ cells from three mice per group. (C) CFSE analysis of the mice in (B) treated at day 0 with DT. Analysis is of gated Ly5.2+ CD8+ tet+ cells. The total number of CFSE-high cells is indicated.
Similar articles
- Preferential localization of effector memory cells in nonlymphoid tissue.
Masopust D, Vezys V, Marzo AL, Lefrançois L. Masopust D, et al. Science. 2001 Mar 23;291(5512):2413-7. doi: 10.1126/science.1058867. Epub 2001 Mar 1. Science. 2001. PMID: 11264538 - XCR1+ dendritic cells promote memory CD8+ T cell recall upon secondary infections with Listeria monocytogenes or certain viruses.
Alexandre YO, Ghilas S, Sanchez C, Le Bon A, Crozat K, Dalod M. Alexandre YO, et al. J Exp Med. 2016 Jan 11;213(1):75-92. doi: 10.1084/jem.20142350. Epub 2015 Dec 22. J Exp Med. 2016. PMID: 26694969 Free PMC article. - Induction and visualization of mucosal memory CD8 T cells following systemic virus infection.
Kim SK, Schluns KS, Lefrançois L. Kim SK, et al. J Immunol. 1999 Oct 15;163(8):4125-32. J Immunol. 1999. PMID: 10510347 - On T cell memory: arguments for antigen dependence.
Kündig TM, Bachmann MF, Ohashi PS, Pircher H, Hengartner H, Zinkernagel RM. Kündig TM, et al. Immunol Rev. 1996 Apr;150:63-90. doi: 10.1111/j.1600-065x.1996.tb00696.x. Immunol Rev. 1996. PMID: 8782702 Review. - Quantitative studies of CD8+ T-cell responses during microbial infection.
Serbina N, Pamer EG. Serbina N, et al. Curr Opin Immunol. 2003 Aug;15(4):436-42. doi: 10.1016/s0952-7915(03)00071-2. Curr Opin Immunol. 2003. PMID: 12900276 Review.
Cited by
- The emerging role of effector functions exerted by tissue-resident memory T cells.
Iijima N. Iijima N. Oxf Open Immunol. 2024 Jun 14;5(1):iqae006. doi: 10.1093/oxfimm/iqae006. eCollection 2024. Oxf Open Immunol. 2024. PMID: 39193473 Free PMC article. Review. - Crosstalking with Dendritic Cells: A Path to Engineer Advanced T Cell Immunotherapy.
Schafer S, Chen K, Ma L. Schafer S, et al. Front Syst Biol. 2024;4:1372995. doi: 10.3389/fsysb.2024.1372995. Epub 2024 Apr 29. Front Syst Biol. 2024. PMID: 38911455 Free PMC article. - A specific and portable gene expression program underlies antigen archiving by lymphatic endothelial cells.
Sheridan RM, Doan TA, Lucas C, Forward TS, Uecker-Martin A, Morrison TE, Hesselberth JR, Tamburini BAJ. Sheridan RM, et al. bioRxiv [Preprint]. 2024 Apr 2:2024.04.01.587647. doi: 10.1101/2024.04.01.587647. bioRxiv. 2024. PMID: 38617225 Free PMC article. Preprint. - Immunization-induced antigen archiving enhances local memory CD8+ T cell responses following an unrelated viral infection.
Doan TA, Forward TS, Schafer JB, Lucas ED, Fleming I, Uecker-Martin A, Ayala E, Guthmiller JJ, Hesselberth JR, Morrison TE, Tamburini BAJ. Doan TA, et al. NPJ Vaccines. 2024 Mar 21;9(1):66. doi: 10.1038/s41541-024-00856-6. NPJ Vaccines. 2024. PMID: 38514656 Free PMC article. - Targeting the dendritic cell-T cell axis to develop effective immunotherapies for glioblastoma.
Gardam B, Gargett T, Brown MP, Ebert LM. Gardam B, et al. Front Immunol. 2023 Oct 20;14:1261257. doi: 10.3389/fimmu.2023.1261257. eCollection 2023. Front Immunol. 2023. PMID: 37928547 Free PMC article. Review.
References
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. - PubMed
- Bachmann MF, Barner M, Viola A, Kopf M. Distinct kinetics of cytokine production and cytolysis in effector and memory T cells after viral infection. Eur. J. Immunol. 1999a;29:291–299. - PubMed
- Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat. Immunol. 2002;3:619–626. - PubMed
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI 41576/AI/NIAID NIH HHS/United States
- R01 DK045260-15/DK/NIDDK NIH HHS/United States
- P01 AI056172/AI/NIAID NIH HHS/United States
- AI 56172/AI/NIAID NIH HHS/United States
- R01 AI041576-10/AI/NIAID NIH HHS/United States
- DK 45260/DK/NIDDK NIH HHS/United States
- P01 AI056172-05/AI/NIAID NIH HHS/United States
- R01 DK045260/DK/NIDDK NIH HHS/United States
- R01 AI041576/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials