Acetylcholine in the orbitofrontal cortex is necessary for the acquisition of a socially transmitted food preference - PubMed (original) (raw)
. 2005 May-Jun;12(3):302-6.
doi: 10.1101/lm.91605. Epub 2005 May 16.
Affiliations
- PMID: 15897258
- PMCID: PMC1142459
- DOI: 10.1101/lm.91605
Acetylcholine in the orbitofrontal cortex is necessary for the acquisition of a socially transmitted food preference
Robert S Ross et al. Learn Mem. 2005 May-Jun.
Abstract
The social transmission of food preference task (STFP) has been used to examine the involvement of the hippocampus in learning and memory for a natural odor-odor association. However, cortical involvement in STFP has not been extensively studied. The orbitofrontal cortex (OFC) is important in odor-guided learning, and cholinergic depletion of the entire neocortex results in impairments in STFP. Here we examined the specific role of cholinergic modulation in the OFC by assessing the effect of 192 immunoglobulin G-saporin infusion directly into OFC prior to training on STFP. Cholinergic depletion in the OFC impaired expression of the socially transmitted odor association measured 2 d after training, indicating that cholinergic function in the OFC is essential for this form of associative learning.
Figures
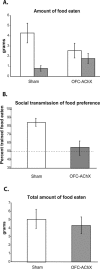
Figure 1.
(A) Amount of trained food eaten (white bar) for each group in grams vs. amount of comparison food eaten (dark bar) during testing period. (B) Performance by OFC-AChX rats (dark bar) and sham operated controls (white bar) in STFP. The dashed line indicates chance selection of the two foods. (C) Total amount of food eaten by each group in grams. Data are represented as mean ± SEM.
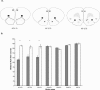
Figure 2.
(A) Highlighted squares (black for orbitofrontal cortex [OFC], gray for cingulate cortex [CIN], and stripes for agranular insular cortex [AI]) represent areas where AChE+ fibers were counted in sham operated controls and in animals with 192 IgG-saporin lesions. Numbers represent AP coordinates relative to bregma (Paxinos and Watson 1998). (B) Bilateral AChE+ fiber counts in all areas examined. Dark bars show counts from OFC-AChX rats, and white bars show counts from sham operated controls. Group labels indicate loci in OFC, CIN, and AI at AP coordinates relative to bregma. Data are represented as mean counts ± SEM. **Significant difference (P < 0.01) in fiber counts between lesion and control group.
Figure 3.
Representative example of AChE depletion seen in the 192 IgG-saporin lesion group in the OFC 2.70 mm anterior to bregma (line = 50 μm).
Similar articles
- The orbitofrontal cortex is not necessary for acquisition or remote recall of socially transmitted food preferences.
Smith CA, East BS, Colombo PJ. Smith CA, et al. Behav Brain Res. 2010 Mar 17;208(1):243-9. doi: 10.1016/j.bbr.2009.12.001. Epub 2009 Dec 11. Behav Brain Res. 2010. PMID: 20004219 - Time-courses of Fos expression in rat hippocampus and neocortex following acquisition and recall of a socially transmitted food preference.
Smith CA, Countryman RA, Sahuque LL, Colombo PJ. Smith CA, et al. Neurobiol Learn Mem. 2007 Jul;88(1):65-74. doi: 10.1016/j.nlm.2007.03.001. Epub 2007 Apr 19. Neurobiol Learn Mem. 2007. PMID: 17448703 - Cholinergic basal forebrain is critical for social transmission of food preferences.
Berger-Sweeney J, Stearns NA, Frick KM, Beard B, Baxter MG. Berger-Sweeney J, et al. Hippocampus. 2000;10(6):729-38. doi: 10.1002/1098-1063(2000)10:6<729::AID-HIPO1010>3.0.CO;2-M. Hippocampus. 2000. PMID: 11153718 - Muscarinic cholinergic receptor blockade in the rat prelimbic cortex impairs the social transmission of food preference.
Boix-Trelis N, Vale-Martínez A, Guillazo-Blanch G, Martí-Nicolovius M. Boix-Trelis N, et al. Neurobiol Learn Mem. 2007 May;87(4):659-68. doi: 10.1016/j.nlm.2006.12.003. Epub 2007 Jan 12. Neurobiol Learn Mem. 2007. PMID: 17223581 - Acetylcholine release in the hippocampus and prelimbic cortex during acquisition of a socially transmitted food preference.
Gold PE, Countryman RA, Dukala D, Chang Q. Gold PE, et al. Neurobiol Learn Mem. 2011 Oct;96(3):498-503. doi: 10.1016/j.nlm.2011.08.004. Epub 2011 Aug 30. Neurobiol Learn Mem. 2011. PMID: 21907814 Free PMC article.
Cited by
- Effects of nucleus basalis magnocellularis stimulation on a socially transmitted food preference and c-Fos expression.
Boix-Trelis N, Vale-Martínez A, Guillazo-Blanch G, Costa-Miserachs D, Martí-Nicolovius M. Boix-Trelis N, et al. Learn Mem. 2006 Nov-Dec;13(6):783-93. doi: 10.1101/lm.305306. Epub 2006 Nov 13. Learn Mem. 2006. PMID: 17101878 Free PMC article. - Assessment of Social Transmission of Food Preferences Behaviors.
Van der Jeugd A, D'Hooge R. Van der Jeugd A, et al. J Vis Exp. 2018 Jan 25;(131):57029. doi: 10.3791/57029. J Vis Exp. 2018. PMID: 29443064 Free PMC article. - Dynamics of hippocampal and cortical activation during consolidation of a nonspatial memory.
Ross RS, Eichenbaum H. Ross RS, et al. J Neurosci. 2006 May 3;26(18):4852-9. doi: 10.1523/JNEUROSCI.0659-06.2006. J Neurosci. 2006. PMID: 16672659 Free PMC article. - Decision making: the neuroethological turn.
Pearson JM, Watson KK, Platt ML. Pearson JM, et al. Neuron. 2014 Jun 4;82(5):950-65. doi: 10.1016/j.neuron.2014.04.037. Neuron. 2014. PMID: 24908481 Free PMC article. Review. - Expression of P301L-hTau in mouse MEC induces hippocampus-dependent memory deficit.
Liu X, Zeng K, Li M, Wang Q, Liu R, Zhang B, Wang JZ, Shu X, Wang X. Liu X, et al. Sci Rep. 2017 Jun 20;7(1):3914. doi: 10.1038/s41598-017-04305-4. Sci Rep. 2017. PMID: 28634382 Free PMC article.
References
- Barbas, H. 2000. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res. Bull. 52: 319-330. - PubMed
- Berger-Sweeney, J., Stearns, N.A., Frick, K.M., Beard, B., and Baxter, M.G. 2000. Cholinergic basal forebrain is critical for social transmission of food preferences. Hippocampus 10: 729-738. - PubMed
- Bigl, V., Woolf, N.J., and Butcher, L.L. 1982. Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: A combined fluorescent tracer and acetylcholinesterase analysis. Brain Res. Bull. 8: 727-749. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources