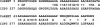Components of the REST/CoREST/histone deacetylase repressor complex are disrupted, modified, and translocated in HSV-1-infected cells - PubMed (original) (raw)
Comparative Study
. 2005 May 24;102(21):7571-6.
doi: 10.1073/pnas.0502658102. Epub 2005 May 16.
Affiliations
- PMID: 15897453
- PMCID: PMC1140450
- DOI: 10.1073/pnas.0502658102
Comparative Study
Components of the REST/CoREST/histone deacetylase repressor complex are disrupted, modified, and translocated in HSV-1-infected cells
Haidong Gu et al. Proc Natl Acad Sci U S A. 2005.
Abstract
The infected cell protein (ICP)0 enables gene expression and the replication of herpes simplex virus (HSV)-1 in cells infected at low multiplicities and enhances the expression of genes introduced into cells by transfection or infection. We report that a short sequence of ICP0 is similar to a sequence in the amino terminus of CoREST, a corepressor that exists in complexes with the repressor REST and histone deacetylases (HDACs) 1 or 2 to repress cellular gene expression. In wild-type-virus-infected cells, HDAC1 dissociates from the CoREST/REST complex, CoREST and HDAC1 are phosphorylated by a process mediated by viral protein kinases, and CoREST and HDAC1 are partially translocated to the cytoplasm. In cells infected with a virus mutant (DeltaICP4), in which ICP0 accumulates, but post-alpha gene expression is blocked, HDAC1 is dissociated from the CoREST/REST complex, but translocation to the cytoplasm does not occur. After infection with a mutant virus from which ICP0 is deleted, the complex remains intact, but, under conditions of productive infection, the complex is partially translocated to the cytoplasm. These results suggest that, at low multiplicities of infection, ICP0 blocks CoREST-mediated silencing of viral genes by dissociation of HDAC1, whereas subsequent modifications and translocation of the components of the complex are the functions of other viral gene products made later in infection.
Figures
Fig. 1.
Alignment of amino acid sequences of ICP0 and CoREST. Identities, 23 of 77 (31%); positives, 43 of 77 (55%). Expect value = 3 × 10–6.
Fig. 2.
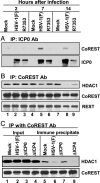
Interactions between ICP0 and the components of the CoREST/REST/HDAC complex. Nuclear lysates from mock-infected, HSV-1(F)-infected, or R7353-infected HeLa cells harvested at 2, 7, or 14 h after infection were reacted with polyclonal Ab against ICP0 (A) or CoREST (B and C). The aliquots of the lysates and immunoprecipitates were electrophoretically separated in denaturing gels and reacted with anti-CoREST, anti-HDAC1, anti-REST, or anti-ICP0 Ab as shown. Input was 10% of the total protein used for immunoprecipitation.
Fig. 3.
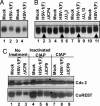
CoREST is phosphorylated in HSV-1(F)-infected cells. (A) Electrophoretically separated lysates of cells harvested 24 h after mock-infection or exposure to SK-N-SH cells were reacted with anti-CoREST Ab as described in Materials and Methods. Duplicate samples from infected cells were interspersed with samples from mock-infected cells to enable better visualization of small changes in electrophoretic mobility. (B) Electrophoretically separated lysates of mock-infected cells and cells infected with HSV-1(F), ΔICP4), ΔUS3, or ΔUL13 mutant viruses were reacted with anti-CoREST Ab. The arrows identify the bands formed by CoREST present in lysates of wild-type-virus-infected cells. (C) Lysates from mock-, HSV-1(F)-, or ΔICP0-mutant-virus-infected cells were reacted with dephosphorylation buffer alone (No treatment), heat-inactivated CIAP, or active CIAP and electrophoretically separated in denaturing gels and reacted to anti-CoREST or anti-cdc2 Ab. The filled circles indicate the disappearance of slower-mobilitybands of cdc2 and CoREST.
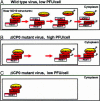
Fig. 4.
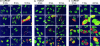
Translocation of CoREST and HDAC1 from nuclei to cytoplasm. (a) HEL fibroblasts grown in four-well slides were fixed 3, 5, or 10 h after infection with HSV-1(F), ΔICP4, or ΔICP0 mutant viruses. The fixed slides were reacted with anti-CoREST (FITC) and anti-ICP0 (Texas red) Abs (A_–_I) or anti-CoREST (FITC) and anti-ICP8 (Texas red) (J_–_L) Abs. Images were collected in a Zeiss confocal microscope equipped with a ×63 objective. (b) Duplicate slides prepared as described for a were reacted with anti-HDAC1 in place of anti-CoREST (FITC) and either anti-ICP0 (M_–_U) or anti-ICP8 (V_–_X) (Texas red). (c) SK-N-SH cells grown on four-well slides were fixed at 3, 7, or 24 h after mock-infection or infection with HSV-1(F) or ΔICP4 mutant virus (A_–_I). One set of wells was infected with wild-type virus and maintained in medium containing phosphonoacetate (PAA, 300 μg/ml) (J_–_L). The cells were reacted with anti-CoREST Ab (FITC) and anti-ICP0 (Texas red). The arrowheads in aE, aF, and aL and in cE and cL point to cytoplasmic CoREST. The arrowheads in bQ, bR, and bV point to cytoplasmic HDAC1. The arrowhead in aI points to cytoplasmic aggregates of ICP0. Similar aggregates are present in corresponding images in b and c.
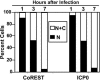
Fig. 5.
Localization of CoREST and ICP0. SK-N-SH cells grown on four-well slides were fixed at 1, 3, or 7 h after mock-infection or infection with HSV-1(F). The localization of ICP0 and CoREST from ≈200 cells for each infection was tabulated under the microscope. The numbers of cells with ICP0 or CoREST present in both nucleus and cytoplasm (N+C) or nucleus (N) are shown as the percentage of total counted cells.
Fig. 6.
Dissociation of HDAC1 from the CoREST/REST complex after HSV-1 infection. Replicated cultures of HeLa cells were mock- or HSV-1(F)-infected for 3 or 12 h and fractionated with either a Dounce homogenizer or nonionic detergents as described in Materials and Methods. The precipitates obtained with the anti-CoREST Ab from the nuclear and cytoplasmic fractions and samples of the lysates were individually electrophoretically separated in denaturing gels and reacted with Ab to HDAC1, REST, or CoREST. The amounts of CoREST, REST, or HDAC1 antigen in the nuclear or cytoplasmic fractions and in the precipitates were quantified with the Molecular Dynamics PhosphorImager Storm 860 fluorescence imager and
imagequest
5 software. The ratios of precipitated REST or HDAC1 to total antigen (Ag) available for precipitation were plotted with
excel
software (Microsoft). Input was 10% of the lysate used for immunoprecipitation.
Fig. 7.
A model of the role of ICP0 in the initiation of viral post-α gene expression. (A) Newly made ICP0 and entering HSV DNA colocalize in the vicinity of ND10 nuclear structures. ICP0 mediates the displacement of HDAC1 from the CoREST/REST complex, enabling post-α gene expression. Post-α gene products mediate the posttranslational modification of HDAC1/2 and CoREST, which are then exported to the cytoplasm. The representation of the HDAC/CoREST/REST repressor complex follows the model proposed by Ballas et al. (9). (B) In cells infected at high ratios of ΔICP0 virions per cell, HDAC/CoREST/REST complex remains intact, but the complex is exported to the cytoplasm. (C) In the absence of post-α gene expression, this process does not occur in cells infected at low ΔICP0 particle-to-cell ratios.
Similar articles
- Engagement of the lysine-specific demethylase/HDAC1/CoREST/REST complex by herpes simplex virus 1.
Gu H, Roizman B. Gu H, et al. J Virol. 2009 May;83(9):4376-85. doi: 10.1128/JVI.02515-08. Epub 2009 Feb 4. J Virol. 2009. PMID: 19193804 Free PMC article. - Herpes simplex virus-infected cell protein 0 blocks the silencing of viral DNA by dissociating histone deacetylases from the CoREST-REST complex.
Gu H, Roizman B. Gu H, et al. Proc Natl Acad Sci U S A. 2007 Oct 23;104(43):17134-9. doi: 10.1073/pnas.0707266104. Epub 2007 Oct 15. Proc Natl Acad Sci U S A. 2007. PMID: 17939992 Free PMC article. - The two functions of herpes simplex virus 1 ICP0, inhibition of silencing by the CoREST/REST/HDAC complex and degradation of PML, are executed in tandem.
Gu H, Roizman B. Gu H, et al. J Virol. 2009 Jan;83(1):181-7. doi: 10.1128/JVI.01940-08. Epub 2008 Oct 22. J Virol. 2009. PMID: 18945770 Free PMC article. - The first 30 minutes in the life of a virus: unREST in the nucleus.
Roizman B, Gu H, Mandel G. Roizman B, et al. Cell Cycle. 2005 Aug;4(8):1019-21. doi: 10.4161/cc.4.8.1902. Epub 2005 Aug 7. Cell Cycle. 2005. PMID: 16082207 Review. - CoREST-like complexes regulate chromatin modification and neuronal gene expression.
Lakowski B, Roelens I, Jacob S. Lakowski B, et al. J Mol Neurosci. 2006;29(3):227-39. doi: 10.1385/JMN:29:3:227. J Mol Neurosci. 2006. PMID: 17085781 Review.
Cited by
- Chromatin dynamics during lytic infection with herpes simplex virus 1.
Conn KL, Schang LM. Conn KL, et al. Viruses. 2013 Jul 16;5(7):1758-86. doi: 10.3390/v5071758. Viruses. 2013. PMID: 23863878 Free PMC article. Review. - Cellular Protein WDR11 Interacts with Specific Herpes Simplex Virus Proteins at the trans-Golgi Network To Promote Virus Replication.
Taylor KE, Mossman KL. Taylor KE, et al. J Virol. 2015 Oct;89(19):9841-52. doi: 10.1128/JVI.01705-15. Epub 2015 Jul 15. J Virol. 2015. PMID: 26178983 Free PMC article. - During lytic infections, herpes simplex virus type 1 DNA is in complexes with the properties of unstable nucleosomes.
Lacasse JJ, Schang LM. Lacasse JJ, et al. J Virol. 2010 Feb;84(4):1920-33. doi: 10.1128/JVI.01934-09. Epub 2009 Dec 9. J Virol. 2010. PMID: 20007274 Free PMC article. - Chromatin dynamics and the transcriptional competence of HSV-1 genomes during lytic infections.
Hu M, Depledge DP, Flores Cortes E, Breuer J, Schang LM. Hu M, et al. PLoS Pathog. 2019 Nov 14;15(11):e1008076. doi: 10.1371/journal.ppat.1008076. eCollection 2019 Nov. PLoS Pathog. 2019. PMID: 31725813 Free PMC article.
References
- Chong, J. A., Tapia-Ramirez, J., Kim, S., Toledo-Aral, J. J., Zheng, Y., Boutros, M. C., Altshuller, Y. M., Frohman, M. A., Kraner, S. D. & Mandel, G. (1995) Cell 80, 949–957. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA78766/CA/NCI NIH HHS/United States
- CA83939/CA/NCI NIH HHS/United States
- R01 CA078766/CA/NCI NIH HHS/United States
- CA71933/CA/NCI NIH HHS/United States
- CA87661/CA/NCI NIH HHS/United States
- P01 CA087661/CA/NCI NIH HHS/United States
- NS22518/NS/NINDS NIH HHS/United States
- P01 CA071933/CA/NCI NIH HHS/United States
- R01 CA088860/CA/NCI NIH HHS/United States
- CA88860/CA/NCI NIH HHS/United States
- R37 CA078766/CA/NCI NIH HHS/United States
- R01 CA083939/CA/NCI NIH HHS/United States
- R01 NS022518/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous