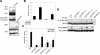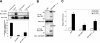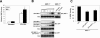The noncatalytic amino terminus of mitogen-activated protein kinase phosphatase 1 directs nuclear targeting and serum response element transcriptional regulation - PubMed (original) (raw)
The noncatalytic amino terminus of mitogen-activated protein kinase phosphatase 1 directs nuclear targeting and serum response element transcriptional regulation
J Julie Wu et al. Mol Cell Biol. 2005 Jun.
Abstract
The mitogen-activated protein kinase (MAPK) phosphatase 1 (MKP-1) is an immediate-early gene comprised of a dual-specificity phosphatase domain and a noncatalytic NH(2) terminus. Here, we show that the NH(2) terminus of MKP-1, containing the cdc25 homology domains A (CH2A) and B (CH2B), mediates MKP-1 nuclear targeting and modulates MAPK-mediated gene expression. An LXXLL motif which is known to mediate protein-protein interactions with nuclear-targeted hormone receptors was identified proximal to the CH2A domain of MKP-1. The NH(2) terminus alone of MKP-1 containing this LXXLL motif was sufficient to direct nuclear targeting, and mutating this motif to LXXAA resulted in the exclusion of MKP-1 from the nucleus. We found that the LXXLL motif proximal to the CH2A domain was present in other nuclear-localized MKPs but was absent in MKPs that localized to the cytoplasm. These data suggest that this LXXLL motif confers nuclear targeting properties to the MKPs. The NH(2) terminus of MKP-1 was also found to inhibit the activation of the serum response element (SRE) by preventing MAPK-mediated phosphorylation of the regulatory serine 383 residue on Elk-1. Moreover, we show that MKP-1 plays a major role in the attenuation of serum-induced SRE activity, since MKP-1 null fibroblasts exhibited enhanced SRE activity in response to serum compared with wild-type fibroblasts. The NH(2) terminus of MKP-1, when reconstituted into MKP-1 null fibroblasts to levels similar to endogenous MKP-1 following serum stimulation, reduced serum-mediated SRE activity. Collectively, these data reveal novel roles for the NH(2) terminus of MKP-1 in nuclear targeting and transcriptional regulation.
Figures
FIG. 1.
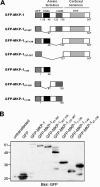
Generation of GFP-MKP-1 fusion proteins. (A) Schematic representation of the GFP fusion proteins of MKP-1 used in this study. (B) COS-7 cells were either left untransfected or were transfected with expression vectors encoding the GFP-MKP-1 fusion proteins shown in panel A. Cell lysates were prepared and subjected to SDS-PAGE followed by immunoblotting with anti-GFP antibodies.
FIG. 2.
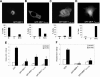
The NH2 terminus of MKP-1 is required for nuclear localization. (A to D) COS-7 cells were transiently transfected with GFP-MKP-1 (A), GFP- MKP-147-367 (B), GFP-MKP-1Δ47-136 (C), or GFP-MKP-1137-367 (D). Confocal imaging was used to visualize GFP expression. The graphs below each photomicrograph in panels A to D represent the quantitation from a representative experiment in which >100 GFP-positive cells were analyzed for each condition. Shown is the percentage of cells in which GFP fluorescence was predominately nuclear (N), cytoplasmic (C), or both (N+C). (E and F) 293 cells were transiently transfected with the indicated GFP-MKP-1 expression plasmids plus Elk-1c-GAL4, 5XGAL4-luciferase, and pRL-Renilla. Cells were serum deprived and restimulated with 10% FBS. Elk-1 activation was measured as described in Materials and Methods. Data are representative of the mean ± standard error of the mean from three to four separate experiments. *, P < 0.05.
FIG. 3.
The NH2 terminus of MKP-1 is sufficient for nuclear targeting. GFP-MKP-11-46 (A), GFP-MKP-147-136 (B), and GFP-MKP-11-136 (C) were transiently transfected into COS-7 cells, and confocal imaging was performed to visualize for GFP. The graphs below each photomicrograph in panels A to C represent the quantitation from a representative experiment in which >100 GFP-positive cells were analyzed for each condition. Shown are the percentages of cells in which GFP fluorescence was either predominately nuclear (N), cytoplasmic (C), or both (N+C).
FIG. 4.
The LXXLL motif proximal to the CH2A domain is required for MKP-1 nuclear targeting. (A) Sequence alignment of the NH2 terminus of MKP-1, MKP-2, PAC-1, B23, and MKP-7 reveals a conserved LXXLL motif. The LXXLL motif is conserved in human, mouse, rat, and Xenopus (xCL100/xMKP-1) MKP-1. (B) Sequences of the LXXLL MKP-1 mutants. (C) The LXXLL motif mutants shown in panel B were expressed transiently in COS-7 cells along with GFP-MKP-1. Lysates were resolved by SDS-PAGE, and GFP expression was detected by immunoblotting with an anti-MKP-1 antibody. (D) GFP-MKP-1 or the MKP-1-LXXLL mutants were transiently expressed in COS-7 cells, and confocal imaging for the expression of GFP was performed. The inset in panel D shows a quantitative analysis of the GFP fluorescence distribution of the GFP-MKP-1(L16/17A) mutant analyzed as described in the legend for Fig. 2.
FIG. 5.
The MAPK-binding site on MKP-1 contributes to nuclear accumulation. (A) Schematic representation of the basic cluster (RRR) on MKP-1 that resides between the CH2A and CH2B domains. This RRR basic cluster was mutated to ASA in the context of GFP-MKP-1 to generate GFP-MKP-1(ASA). (B) Expression of GFP-MKP-1(ASA) was confirmed by transient transfection into COS-7 cells followed by immunoblot analysis using anti-MKP-1 antibodies. (C) Confocal imaging for GFP expression in COS-7 cells transiently expressing GFP, GFP-MKP-1, or GFP-(MKP-1)ASA proteins. The inset in panel C shows a graphical representation of the GFP fluorescence distribution of the GFP-MKP-1(ASA) mutant analyzed as described in the legend for Fig. 2.
FIG. 6.
The NH2 terminus of MKP-1 inhibits MAPK-mediated Elk-1 activation. (A) Serum-deprived 293 cells were either left untreated or were restimulated with 10% FBS for 30 min. Protein lysates were resolved by SDS-PAGE and immunoblotted with anti-phospho-specific antibodies to Erk1/2 (pErk), p38 MAPK (pp38 MAPK), and JNK (pJNK). Immunoblots were probed with anti-Erk, p38 MAPK, and JNK antibodies. Anisomycin-treated cells (5 μM anisomycin for 1 h) served as a positive control for JNK activation. (B) 293 cells were cotransfected with the Elk-1 reporter genes, and Elk-1 activation was measured as described in the legend for Fig. 2. 293 cells were pretreated with either dimethyl sulfoxide, PD098059 (5 μM), or SB203580 (10 μM) prior to stimulation with 10% FBS for 4 h. Data represent the means ± standard errors of the means (SEM) of three separate experiments. (C) The indicated GFP-MKP-1 fusion proteins (Fig. 1A) were expressed with the Elk-1 reporter genes in 293 cells, and Elk-1 activity was determined either in the absence or presence of 10% FBS stimulation. The data are represented as the mean ± SEM change in Elk-1 activation from four separate experiments performed in triplicate. *, P < 0.05. (D) 293 cells were cotransfected with the indicated GFP-MKP-1 fusion proteins along with HA-Erk2. Transfectants were serum deprived and were then either left untreated or restimulated with 10% FBS for 30 min. Anti-HA-Erk2 immunoprecipitates were immunoblotted with phospho-Erk1/2 antibodies (upper panel). The immunoblot was reprobed with anti-Erk1/2 antibodies (lower panel). Whole-cell lysates were prepared and analyzed for p38 MAPK activation using anti-phospho-p38 MAPK (upper panel) and p38 MAPK (lower panel) antibodies.
FIG. 7.
The NH2 terminus of MKP-1 inhibits SRE-mediated activation by preventing Elk-1 phosphorylation. (A) Serum-deprived 293 cells expressing the indicated GFP-MKP-1 fusion proteins along with Flag-Elk-1 were stimulated with 10% FBS for 30 min. Whole-cell lysates prepared from these transfectants were resolved by SDS-PAGE and immunoblotted with either anti-phospho-Elk-1 (Ser 383) or anti-Flag antibodies. The graph below represents the means ± standard errors of the means (SEM) of three separate experiments performed as described above, in which densitometric analyses were determined from phospho-Elk-1 and Elk-1 immunoblots. The phospho-Elk-1/Elk-1 ratio as a percentage of serum-induced Elk-1 phosphorylation upon GFP-MKP-1 and GFP-MKP-11-136 expression relative to GFP is shown. (B) 293 cells were transfected with HA-Erk2 (upper panel) or Flag-p38 MAPK (lower panel) with either GFP control or GFP-MKP-11-136. HA-Erk2 and Flag-p38 MAPK immune complexes were resolved and immunoblotted using anti-GFP antibodies. The lower panels represent controls for Erk (upper panel) and p38 MAPK (lower panel) using anti-Erk2 and p38 MAPK, respectively. (C) 293 cells were cotransfected with 5XSRE-luciferase reporter along with pRL-Renilla and GFP, GFP-MKP-1, and GFP-MKP-11-136. Transfectants were serum deprived and were either left untreated or restimulated with 10% FBS for ∼4 h. SRE luciferase activity was normalized to Renilla units and expressed as the relative SRE activity. The data shown are representative of the means ± SEM from four separate experiments. *, P < 0.05.
FIG. 8.
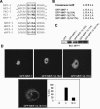
MKP-1 and its NH2 terminus are important for the inactivation of SRE-mediated transcriptional activity. (A) Primary wild-type MKP-1 (MKP-1+/+) and MKP-1 null (MKP-1−/−) MEFs were serum deprived for 48 h and restimulated with 10% FBS for ∼4 h. SRE luciferase activity was normalized to Renilla units and expressed as the relative fold SRE activity. The data shown are representative of the means ± standard errors of the means (SEM) from five separate experiments. *, P < 0.05. (B) Immortalized MKP-1−/− MEFs were transiently transfected with the indicated amounts of MKP-1 expression plasmid, or MKP-1+/+ MEFs were left untreated or were stimulated with 10% FBS for 1 h. Cells were lysed, resolved by SDS-PAGE, and immunoblotted with anti-MKP-1 antibodies. Expression of the GFP fusion proteins was determined by immunoblotting cell lysates with anti-GFP antibodies. (C) MKP-1−/− MEFs were transfected with 5XSRE-luciferase reporter along with pRL-Renilla and either GFP, GFP-MKP-1, or GFP-MKP-11-136. Cells were stimulated with 10% FBS for ∼4 h, and SRE luciferase activity was normalized to Renilla units. Results are shown as percent SRE activity relative to the GFP control and represent the means ± SEM from four separate experiments.
Similar articles
- The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo.
Davis S, Vanhoutte P, Pages C, Caboche J, Laroche S. Davis S, et al. J Neurosci. 2000 Jun 15;20(12):4563-72. doi: 10.1523/JNEUROSCI.20-12-04563.2000. J Neurosci. 2000. PMID: 10844026 Free PMC article. - Regulation of c-Jun N-terminal kinase and p38 kinase pathways in endothelial cells.
Wadgaonkar R, Pierce JW, Somnay K, Damico RL, Crow MT, Collins T, Garcia JG. Wadgaonkar R, et al. Am J Respir Cell Mol Biol. 2004 Oct;31(4):423-31. doi: 10.1165/rcmb.2003-0384OC. Epub 2004 Jul 1. Am J Respir Cell Mol Biol. 2004. PMID: 15231489 - Regulation of innate immune response by MAP kinase phosphatase-1.
Wang X, Liu Y. Wang X, et al. Cell Signal. 2007 Jul;19(7):1372-82. doi: 10.1016/j.cellsig.2007.03.013. Epub 2007 Apr 20. Cell Signal. 2007. PMID: 17512700 Free PMC article. Review. - Protein tyrosine phosphatases: mechanisms of catalysis and regulation.
Denu JM, Dixon JE. Denu JM, et al. Curr Opin Chem Biol. 1998 Oct;2(5):633-41. doi: 10.1016/s1367-5931(98)80095-1. Curr Opin Chem Biol. 1998. PMID: 9818190 Review.
Cited by
- Regulation of mitochondrial functions by protein phosphorylation and dephosphorylation.
Lim S, Smith KR, Lim ST, Tian R, Lu J, Tan M. Lim S, et al. Cell Biosci. 2016 Apr 14;6:25. doi: 10.1186/s13578-016-0089-3. eCollection 2016. Cell Biosci. 2016. PMID: 27087918 Free PMC article. Review. - Dexamethasone inhibits the Nox-dependent ROS production via suppression of MKP-1-dependent MAPK pathways in activated microglia.
Huo Y, Rangarajan P, Ling EA, Dheen ST. Huo Y, et al. BMC Neurosci. 2011 May 26;12:49. doi: 10.1186/1471-2202-12-49. BMC Neurosci. 2011. PMID: 21615929 Free PMC article. - Genkwanin inhibits proinflammatory mediators mainly through the regulation of miR-101/MKP-1/MAPK pathway in LPS-activated macrophages.
Gao Y, Liu F, Fang L, Cai R, Zong C, Qi Y. Gao Y, et al. PLoS One. 2014 May 6;9(5):e96741. doi: 10.1371/journal.pone.0096741. eCollection 2014. PLoS One. 2014. PMID: 24800851 Free PMC article. - Mice lacking MKP-1 and MKP-5 Reveal Hierarchical Regulation of Regenerative Myogenesis.
Shi H, Gatzke F, Molle JM, Lee HB, Helm ET, Oldham JJ, Zhang L, Gerrard DE, Bennett AM. Shi H, et al. J Stem Cell Regen Biol. 2015 Nov 12;1(1):1-7. doi: 10.15436/2741-0598.15.005. J Stem Cell Regen Biol. 2015. PMID: 27064463 Free PMC article. - Regulation of cardiac hypertrophy and remodeling through the dual-specificity MAPK phosphatases (DUSPs).
Liu R, Molkentin JD. Liu R, et al. J Mol Cell Cardiol. 2016 Dec;101:44-49. doi: 10.1016/j.yjmcc.2016.08.018. Epub 2016 Aug 27. J Mol Cell Cardiol. 2016. PMID: 27575022 Free PMC article. Review.
References
- Brondello, J.-M., A. Brunet, J. Pouyssegur, and F. R. McKenzie. 1996. The dual specificity mitogen-activated protein kinase phosphatase-1 and -2 are induced by the p42/p44MAPK cascade. J. Biol. Chem. 272:1368-1376. - PubMed
- Camps, M., A. Nichols, and S. Arkinstall. 2000. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J. 14:6-16. - PubMed
- Camps, M., A. Nichols, C. Gillieron, B. Antonsson, M. Muda, C. Chabert, U. Boschert, and S. Arkinstall. 1998. Catalytic activation of the phosphatase MKP-3 by ERK2 mitogen-activated protein kinase. Science 280:1262-1264. - PubMed
- Charles, C. H., A. S. Abler, and L. F. Lau. 1992. cDNA sequence of a growth-inducible immediate early gene and characterization of its encoded protein. Oncogene 7:187-190. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous