Regulation of NMDA receptors by neuregulin signaling in prefrontal cortex - PubMed (original) (raw)
Comparative Study
Regulation of NMDA receptors by neuregulin signaling in prefrontal cortex
Zhenglin Gu et al. J Neurosci. 2005.
Abstract
Recent linkage studies have identified a significant association of the neuregulin gene with schizophrenia, but how neuregulin is involved in schizophrenia is primarily unknown. Aberrant NMDA receptor functions have been implicated in the pathophysiology of schizophrenia. Therefore, we hypothesize that neuregulin, which is present in glutamatergic synaptic vesicles, may affect NMDA receptor functions via actions on its ErbB receptors enriched in postsynaptic densities, hence participating in emotional regulation and cognitive processes that are impaired in schizophrenia. To test this, we examined the regulation of NMDA receptor currents by neuregulin signaling pathways in prefrontal cortex (PFC), a prominent area affected in schizophrenia. We found that bath perfusion of neuregulin significantly reduced whole-cell NMDA receptor currents in acutely isolated and cultured PFC pyramidal neurons and decreased NMDA receptor-mediated EPSCs in PFC slices. The effect of neuregulin was mainly blocked by application of the ErbB receptor tyrosine kinase inhibitor, phospholipase C (PLC) inhibitor, IP3 receptor (IP3R) antagonist, or Ca2+ chelators. The neuregulin regulation of NMDA receptor currents was also markedly attenuated in cultured neurons transfected with mutant forms of Ras or a dominant-negative form of MEK1 (mitogen-activated protein kinase kinase 1). Moreover, the neuregulin effect was prevented by agents that stabilize or disrupt actin polymerization but not by agents that interfere with microtubule assembly. Furthermore, neuregulin treatment increased the abundance of internalized NMDA receptors in cultured PFC neurons, which was also sensitive to agents affecting actin cytoskeleton. Together, our study suggests that both PLC/IP3R/Ca2+ and Ras/MEK/ERK (extracellular signal-regulated kinase) signaling pathways are involved in the neuregulin-induced reduction of NMDA receptor currents, which is likely through enhancing NR1 internalization via an actin-dependent mechanism.
Figures
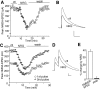
Figure 1.
Neuregulin reversibly reduced NMDA receptor currents in PFC pyramidal neurons. A, Plot of peak NMDAR current showing that bath application of the polypeptide containing the EGF domain of NRG-β1 (NRG; 4 n
m
) decreased NMDA (100 μ
m
)-elicited ionic currents in a dissociated neuron. B, Representative current traces taken from the records used to construct A (at time points denoted by #). Calibration: 0.2 nA, 1 s. C, Dose-response data showing the percentage of reduction (reduc.) of NMDAR currents (curr.) by different concentrations of the EGF domain of NRG-β1. Error bars represent SEM. D, Plot of normalized peak NMDAR currents showing that the broad-spectrum tyrosine kinase inhibitor genistein (20 μ
m
) or the more specific ErbB inhibitor PD158780 (1 μ
m
) mainly blocked the NRG reduction of NMDAR currents. E, Cumulative data (mean ± SEM) showing the percentage reduction (reduc.) of NMDAR currents (curr.) by NRG (4 n
m
) in the absence (-) or presence of genistein or PD158780. *p < 0.01; ANOVA; compared with (-). ctl, Control. Error bars represent SEM.
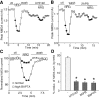
Figure 2.
NRG reduced the amplitude, but not the paired-pulse ratio, of NMDAR EPSCs in PFC slices. A, Plot of peak NMDAR EPSCs showing that NRG (4 n
m
) application reversibly reduced the amplitude of NMDAR-mediated synaptic responses. B, Representative current traces (average of 3 trials) taken from the records used to construct A (at time points denoted by #). Calibration: 50 pA, 0.1 s. C, Plot of peak NMDAR EPSCs evoked by double pulses (interstimuli interval, 100 ms) as a function of time and NRG application. D, Representative traces of NMDAR EPSCs (average of 3 trials) taken from the records used to construct C (at time points denoted by #). Calibration: 50 pA, 0.1 s. E, Cumulative data (mean ± SEM) showing the percentage of change of NMDAR EPSC amplitude and PPR by NRG. ctl, Control.
Figure 3.
Inhibition of the PLC/IP3/Ca2+ pathway blocked the NRG reduction of NMDAR currents. A, B, Plot of peak NMDAR currents showing that the PLC inhibitor U73122 (1 μ
m
; A) or the IP3 receptor antagonist 2APB (15 μ
m
; B) essentially blocked the NRG (4 n
m
) effect on NMDAR currents. C, Plot of normalized peak NMDAR currents showing that dialysis with the high BAPTA (10 m
m
) internal solution blocked the NRG effect on NMDAR currents. D, Cumulative data (mean ± SEM) showing the percentage reduction (reduc.) of NMDAR currents (curr.) by NRG under various treatments. *p < 0.01; ANOVA; compared with (-). ctl, Control.
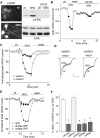
Figure 4.
The Ras/MEK/ERK pathway was involved in the NRG reduction of NMDAR currents. A, Immunocytochemical staining (left) and Western blotting (right) showing that application of NRG (4 n
m
, 2 min) markedly increased phosphorylated ERK (p-ERK) in cultured PFC neurons. Incubation with the MEK inhibitor U0126 (20 μ
m
, 5 min) abolished the NRG-induced increase of p-ERK. B, Plot of peak NMDAR currents showing that U0126 (20 μ
m
) prevented NRG from suppressing NMDAR currents. C, Plot of normalized peak NMDAR currents as a function of time and NRG application in GFP-positive neurons cotransfected with either dnMEK1 or wtMEK1. D, Representative current traces taken from the records used to construct C (at time points denoted by #). Calibration: 0.2 nA, 1 s. E, Plot of normalized peak NMDAR currents as a function of time and NRG application in neurons transfected with GFP alone, GFP-tagged dnRas, or GFP-tagged caRas. F, Cumulative data (mean ± SEM) showing the percentage reduction (reduc.) of NMDAR currents (curr.) by NRG under various conditions. *p < 0.01; ANOVA; compared with (-). ctl, Control.
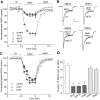
Figure 5.
The NRG effect on NMDAR currents was dependent on actin cytoskeleton but not microtubule. A, Plot of normalized peak NMDAR currents showing that the NRG effect on NMDA currents was markedly attenuated by the actin-depolymerizing agent latrunculin B (5 μ
m
) or cytochalasin D (5 μ
m
) and by the actin stabilizer phalloidin (2 μ
m
; added to the internal solution). B, Representative current traces taken from the records used to construct A (at time points denoted by #). Calibration: 0.2 nA, 1 s. C, Plot of normalized peak NMDAR currents showing that the microtubule depolymerizer colchicine (30 μ
m
) or the microtubule stabilizer Taxol (10 μ
m
) failed to affect the NRG modulation of NMDAR currents. D, Cumulative data (mean ± SEM) showing the percentage of reduction (reduc.) of NMDAR currents (curr.) by NRG in the presence of various chemicals that interfere with actin or microtubule stability. *p < 0.01; ANOVA; compared with (-). ctl, Control; phall, phalloidin; cochi, colchicine.
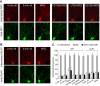
Figure 6.
NRG treatment increased NR1 internalization in cultured PFC neurons. A, Immunocytochemical images showing the staining of internalized and total NR1 in representative neurons treated without or with NRG (5 min) in the absence or presence of various agents (20 min preincubation), including the ErbB kinase inhibitor PD158780 (1 μ
m
), PLC inhibitor U73122 (1 μ
m
), or MEK inhibitor U0126 (20 μ
m
). B, Immunocytochemical images showing the staining of internalized and total GluR1 in representative neurons treated without or with NRG. C, Quantitation of internalized, total, and internalized (intern.)/total ratio of NR1 or GluR1 in neurons treated without or with NRG in the absence or presence of various agents. Values are expressed as a percentage of the 0 min control of total NR1 or GluR1. U73, U73122. *p < 0.01; ANOVA; compared with control. Error bars represent SEM. ctl, Control.
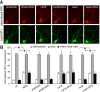
Figure 7.
The NRG-induced increase of NR1 internalization was via an actin-dependent mechanism. A, Immunocytochemical images showing the staining of internalized and total NR1 in representative neurons treated without or with NRG (5 min) in the presence of various agents (1 h preincubation), including the actin stabilizer phalloidin oleate (0.5 μ
m
), the actin depolymerizer latrunculin B (5 μ
m
), or the microtubule stabilizer Taxol (10 μ
m
). B, Quantitation of internalized, total, and internalized (intern.)/total ratio of NR1 in neurons treated without or with NRG in the absence or presence of various agents. Values are expressed as a percentage of the 0 min control (ctl) of total NR1. phall, Phalloidin. *p < 0.01; ANOVA. Error bars represent SEM.
Figure 8.
The NRG reduction of NMDAR currents was through a mechanism involving the NMDAR internalization mediated by a clathrin/dynamin-dependent pathway. A, Plot of normalized (Norm.) peak NMDAR currents as a function of time and NRG application in neurons dialyzed with the dynamin inhibitory (inh.) peptide (pep; 50 μ
m
) or a scrambled control peptide (50 μ
m
). ctl, Control. B, Cumulative data (mean ± SEM) showing the percentage of reduction (reduc.) of NMDAR currents (curr.) by NRG (4 n
m
) in neurons injected with the dynamin inhibitory (inh.) peptide (pep.) or a scrambled (scram.) control peptide. *p < 0.01; ANOVA.
Similar articles
- Regulation of NMDA receptors by dopamine D4 signaling in prefrontal cortex.
Wang X, Zhong P, Gu Z, Yan Z. Wang X, et al. J Neurosci. 2003 Oct 29;23(30):9852-61. doi: 10.1523/JNEUROSCI.23-30-09852.2003. J Neurosci. 2003. PMID: 14586014 Free PMC article. - D2-class dopamine receptor inhibition of NMDA currents in prefrontal cortical neurons is platelet-derived growth factor receptor-dependent.
Beazely MA, Tong A, Wei WL, Van Tol H, Sidhu B, MacDonald JF. Beazely MA, et al. J Neurochem. 2006 Sep;98(5):1657-63. doi: 10.1111/j.1471-4159.2006.04064.x. Epub 2006 Jul 31. J Neurochem. 2006. PMID: 16879713 - Dopamine D1 receptors co-distribute with N-methyl-D-aspartic acid type-1 subunits and modulate synaptically-evoked N-methyl-D-aspartic acid currents in rat basolateral amygdala.
Pickel VM, Colago EE, Mania I, Molosh AI, Rainnie DG. Pickel VM, et al. Neuroscience. 2006 Oct 27;142(3):671-90. doi: 10.1016/j.neuroscience.2006.06.059. Epub 2006 Aug 14. Neuroscience. 2006. PMID: 16905271 - Brain-derived neurotrophic factor regulation of N-methyl-D-aspartate receptor-mediated synaptic currents in suprachiasmatic nucleus neurons.
Kim YI, Choi HJ, Colwell CS. Kim YI, et al. J Neurosci Res. 2006 Nov 15;84(7):1512-20. doi: 10.1002/jnr.21063. J Neurosci Res. 2006. PMID: 16983663 Free PMC article. - Effects of asenapine on prefrontal N-methyl-D-aspartate receptor-mediated transmission: involvement of dopamine D1 receptors.
Jardemark K, Marcus MM, Shahid M, Svensson TH. Jardemark K, et al. Synapse. 2010 Nov;64(11):870-4. doi: 10.1002/syn.20803. Synapse. 2010. PMID: 20842721
Cited by
- Reversal of impaired hippocampal long-term potentiation and contextual fear memory deficits in Angelman syndrome model mice by ErbB inhibitors.
Kaphzan H, Hernandez P, Jung JI, Cowansage KK, Deinhardt K, Chao MV, Abel T, Klann E. Kaphzan H, et al. Biol Psychiatry. 2012 Aug 1;72(3):182-90. doi: 10.1016/j.biopsych.2012.01.021. Epub 2012 Mar 3. Biol Psychiatry. 2012. PMID: 22381732 Free PMC article. - Association study of neuregulin-1 gene polymorphisms in a North Indian schizophrenia sample.
Kukshal P, Bhatia T, Bhagwat AM, Gur RE, Gur RC, Deshpande SN, Nimgaonkar VL, Thelma BK. Kukshal P, et al. Schizophr Res. 2013 Mar;144(1-3):24-30. doi: 10.1016/j.schres.2012.12.017. Epub 2013 Jan 26. Schizophr Res. 2013. PMID: 23360725 Free PMC article. - Advances in the Treatment of Cognitive Impairment in Schizophrenia: Targeting NMDA Receptor Pathways.
Zhang T, Liu C, Zhong N, Wang Y, Huang Y, Zhang X. Zhang T, et al. Int J Mol Sci. 2024 Oct 3;25(19):10668. doi: 10.3390/ijms251910668. Int J Mol Sci. 2024. PMID: 39408997 Free PMC article. Review. - Regulation of the NMDA receptor-mediated synaptic response by acetylcholinesterase inhibitors and its impairment in an animal model of Alzheimer's disease.
Chen G, Chen P, Tan H, Ma D, Dou F, Feng J, Yan Z. Chen G, et al. Neurobiol Aging. 2008 Dec;29(12):1795-804. doi: 10.1016/j.neurobiolaging.2007.04.023. Epub 2007 Jun 6. Neurobiol Aging. 2008. PMID: 17555845 Free PMC article. - An acute effect of neuregulin 1 beta to suppress alpha 7-containing nicotinic acetylcholine receptors in hippocampal interneurons.
Chang Q, Fischbach GD. Chang Q, et al. J Neurosci. 2006 Nov 1;26(44):11295-303. doi: 10.1523/JNEUROSCI.1794-06.2006. J Neurosci. 2006. PMID: 17079657 Free PMC article.
References
- Anton ES, Marchionni MA, Lee KF, Rakic P (1997) Role of GGF/neuregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development 124: 3501-3510. - PubMed
- Buonanno A, Fischbach GD (2001) Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol 11: 287-296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS48911/NS/NINDS NIH HHS/United States
- AG21923/AG/NIA NIH HHS/United States
- R01 NS048911/NS/NINDS NIH HHS/United States
- R01 AG021923/AG/NIA NIH HHS/United States
- R01 MH063128/MH/NIMH NIH HHS/United States
- MH63128/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous