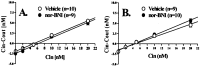Endogenous kappa-opioid receptor systems regulate mesoaccumbal dopamine dynamics and vulnerability to cocaine - PubMed (original) (raw)
Comparative Study
Endogenous kappa-opioid receptor systems regulate mesoaccumbal dopamine dynamics and vulnerability to cocaine
Vladimir I Chefer et al. J Neurosci. 2005.
Abstract
Genetic and pharmacological approaches were used to examine kappa-opioid receptor (KOR-1) regulation of dopamine (DA) dynamics in the nucleus accumbens and vulnerability to cocaine. Microdialysis revealed that basal DA release and DA extraction fraction (Ed), an indirect measure of DA uptake, are enhanced in KOR-1 knock-out mice. Analysis of DA uptake revealed a decreased Km but unchanged Vmax in knock-outs. Knock-out mice exhibited an augmented locomotor response to cocaine, which did not differ from that of wild-types administered a behavioral sensitizing cocaine treatment. The ability of cocaine to increase DA was enhanced in knock-outs, whereas c-fos induction was decreased. Although repeated cocaine administration to wild types produced behavioral sensitization, knock-outs exhibited no additional enhancement of behavior. Administration of the long-acting KOR antagonist nor-binaltorphimine to wild-type mice increased DA dynamics. However, the effects varied with the duration of KOR-1 blockade. Basal DA release was increased whereas Ed was unaltered after 1 h blockade. After 24 h, release and Ed were increased. The behavioral and neurochemical effects of cocaine were enhanced at both time points. These data demonstrate the existence of an endogenous KOR-1 system that tonically inhibits mesoaccumbal DA neurotransmission. Its loss induces neuroadaptations characteristic of "cocaine-sensitized" animals, indicating a critical role of KOR-1 in attenuating responsiveness to cocaine. The increased DA uptake after pharmacological inactivation or gene deletion highlights the plasticity of mesoaccumbal DA neurons and suggests that loss of KOR-1 and the resultant disinhibition of DA neurons trigger short- and long-term DA transporter adaptations that maintain normal DA levels, despite enhanced release.
Figures
Figure 1.
Basal DA dynamics in the NAc of WT, HET, and KOR-1 KO mice. A, Plot of the average gain or loss of DA (_C_in - _C_out) to or from perfusate and the average linear regression fit of the data from no-net-flux microdialysis for each experimental group. The slope of the regression line represents the _E_d. The point when no DA is gained or lost from the perfusate (_C_in - _C_out = 0) represents an unbiased estimate of DAext concentration. The dotted line represents the average regression line for KO mice and has a significantly greater slope relative to WT controls (solid line). B, Bar graph of basal dialysate levels (nanomolar) expressed as means ± SEM; n indicates number of animals per experimental group. * indicates significant difference in dialysate DA levels between WT and KO animals (Newman-Keuls multiple comparison test). C, Bar graph of basal DAext (nanomolar) expressed as means ± SEM. ANOVA revealed no significant difference in this parameter between genotypes (F(2,47) = 0.83; p = 0.44). D, Bar graph of _E_d expressed as the mean ± SEM. * denotes significant difference in _E_d (Newman-Keuls multiple comparison test).
Figure 2.
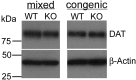
Unaltered levels of DAT in KOR-1 KO mice of mixed (C57BL/6J × 129S6) and congenic (C57BL/6J) backgrounds are shown by Western blot analysis. For quantification, the intensity of each DAT band was normalized to the β-actin band for each lane. The mean relative density and SEM were calculated for each pair. Comparisons between genotypes were made using an unpaired t test with Welch's correction. For mixed background, the mean relative density for WT is 0.73 ± 0.21 versus a mean of 0.76 ± 0.07 for KO (p = 0.914). For the congenic background, WT has a mean of 0.65 ± 0.02 versus KO with a mean of 0.79 ± 0.13 (p = 0.468). No statistically significant differences were seen. The immunoblots are representative comparisons.
Figure 3.
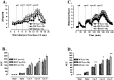
Basal and cocaine-induced locomotor activity and DA levels in the NAc in WT, HET, and KOR-1 KO mice. A, Time course of dialysate DA levels in the NAc in WT (filled squares), HET (filled triangles), and KOR-1 KO (open circles) mice. Each point represents the mean ± SEM; n indicates the number of animals per experimental group. Abscissa, Microdialysis fractions (15 min) before and after cocaine injections. Vertical dotted lines correspond to the time of saline (sal) and cocaine (coc) (5, 10, and 15 mg/kg) injections. B, AUC values for cocaine-evoked DA levels expressed as means ± SEM. * denotes significant difference between WT and KO mice. C, The time course of locomotor activity before and after cocaine challenge in WT (filled squares), HET (filled triangles), and KOR-1 KO (open circles) mice. Each point represents the mean ± SEM; n indicates the number of animals per experimental group. Abscissa, Time (minutes). Vertical dotted lines represent the time of saline and cocaine (5, 10, and 15 mg/kg) injections. D, AUC values for cocaine-evoked locomotor expressed as means ± SEM. * denotes significant difference between WT and KO mice. ** denotes significant difference between HET and KO mice.
Figure 4.
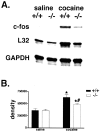
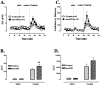
Induction of IEGs after acute cocaine challenge in WT and KOR-1 KO mice. A, A representative RPA gel shows protected fragments from WT and KOR-1 KO mice after acute injection of saline or cocaine (20 mg/kg). The housekeeping genes L32 and GAPDH were used to quantitate levels of RNA per lane. Quantitative decreases in induction of c-fos were observed in KOR-1 KO animals after this dose of cocaine. B, c-fos is significantly increased in both WT and KOR-1 KO mice after cocaine injection, but the induction is significantly less robust in KOR-1 KO mice. * denotes significant difference between saline- and cocaine-treated animals; # denotes significant difference between WT (filled bars) and KO (open bars) mice.
Figure 5.
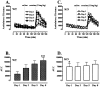
Development of cocaine-induced behavioral sensitization in WT but not KOR-1 KO mice. A, C, Time course of locomotor activity in WT (A) and KO (C) animals (C57BL/6J background) before and after intraperitoneal injections of saline (0.1 ml/10 g) and cocaine (15 mg/kg) on different treatment days. Each point represents the mean ± SEM. Ordinate, Ambulatory distance. Abscissa, Time (minutes). Vertical dotted lines represent the time of saline and cocaine injections. B, D, AUC values for cocaine-evoked locomotor activity response expressed as means ± SEM. * denotes significant difference between WT and KO mice. ** denotes significant difference between the first and the last day of treatment in WT mice.
Figure 6.
Influence of acute or prolonged KOR-1 blockade on basal NAc DA dynamics. DA dynamics were quantified 1 h (A) and 24 h (B) after pretreatment with nor-BNI. A, B, Plots of the average gain or loss of DA (_C_in - _C_out) to or from perfusate and the average linear regression fit of the data from no-net-flux microdialysis for each experimental group. The slope of the regression line represents the _E_d. The point when no DA is gained or lost from the perfusate (_C_in - _C_out = 0) represents an unbiased estimate of DAext concentration.
Figure 7.
DA levels in the NAc and locomotor activity response to an acute cocaine challenge. A, Time course of dialysate DA levels in the NAc in control (open squares) and nor-BNI-treated (filled squares) animals. Each point represents the mean ± SEM; n indicates the number of animals per experimental group. Abscissa, Microdialysis fractions (10 min) before and after (0.1 ml/10 g, i.p.) saline and cocaine (15 mg/kg, i.p.) injections. Vertical dotted lines represent the time of saline and cocaine injections. B, AUC values for cocaine-evoked DA dialysate levels expressed as means ± SEM. * denotes significant difference between control and nor-BNI-treated animals. C, The time course of locomotor activity before and after cocaine challenge in control (open circles) and nor-BNI-treated (filled circles) animals. Each point represents the mean ± SEM; n indicates the number of animals per experimental group. Ordinate, Ambulatory distance. Abscissa, Time (minutes). Vertical dotted lines represent the time of saline and cocaine injections. D, AUC values for cocaine-evoked locomotor activity expressed as means ± SEM. * denotes significant difference between control and nor-BNI-treated animals.
Similar articles
- Paradoxical effects of prodynorphin gene deletion on basal and cocaine-evoked dopaminergic neurotransmission in the nucleus accumbens.
Chefer VI, Shippenberg TS. Chefer VI, et al. Eur J Neurosci. 2006 Jan;23(1):229-38. doi: 10.1111/j.1460-9568.2005.04525.x. Eur J Neurosci. 2006. PMID: 16420432 - Endogenous kappa opioid receptor systems modulate the responsiveness of mesoaccumbal dopamine neurons to ethanol.
Zapata A, Shippenberg TS. Zapata A, et al. Alcohol Clin Exp Res. 2006 Apr;30(4):592-7. doi: 10.1111/j.1530-0277.2006.00069.x. Alcohol Clin Exp Res. 2006. PMID: 16573576 - Increased responsiveness of mesolimbic and mesostriatal dopamine neurons to cocaine following repeated administration of a selective kappa-opioid receptor agonist.
Heidbreder CA, Schenk S, Partridge B, Shippenberg TS. Heidbreder CA, et al. Synapse. 1998 Nov;30(3):255-62. doi: 10.1002/(SICI)1098-2396(199811)30:3<255::AID-SYN3>3.0.CO;2-A. Synapse. 1998. PMID: 9776129 - Neurokinin3 receptor modulation of the behavioral and neurochemical effects of cocaine in rats and monkeys.
Silva MA, Jocham G, Barros M, Tomaz C, Müller CP. Silva MA, et al. Rev Neurosci. 2008;19(2-3):101-11. doi: 10.1515/revneuro.2008.19.2-3.101. Rev Neurosci. 2008. PMID: 18751518 Review. - Modulation of the behavioral and neurochemical effects of psychostimulants by kappa-opioid receptor systems.
Shippenberg TS, Chefer VI, Zapata A, Heidbreder CA. Shippenberg TS, et al. Ann N Y Acad Sci. 2001 Jun;937:50-73. doi: 10.1111/j.1749-6632.2001.tb03558.x. Ann N Y Acad Sci. 2001. PMID: 11458540 Review.
Cited by
- A Possible Anti-anxiety Effect of Appetitive Aggression and a Possible Link to the Work of Donald Winnicott.
Grillo L. Grillo L. Scand J Child Adolesc Psychiatr Psychol. 2022 Aug 30;10(1):102-113. doi: 10.2478/sjcapp-2022-0011. eCollection 2022 Jan. Scand J Child Adolesc Psychiatr Psychol. 2022. PMID: 36133733 Free PMC article. - 15 years of genetic approaches in vivo for addiction research: Opioid receptor and peptide gene knockout in mouse models of drug abuse.
Charbogne P, Kieffer BL, Befort K. Charbogne P, et al. Neuropharmacology. 2014 Jan;76 Pt B(0 0):204-17. doi: 10.1016/j.neuropharm.2013.08.028. Epub 2013 Sep 10. Neuropharmacology. 2014. PMID: 24035914 Free PMC article. Review. - Basolateral amygdala-driven augmentation of medial prefrontal cortex GABAergic neurotransmission in response to environmental stimuli associated with cocaine administration.
Chefer VI, Wang R, Shippenberg TS. Chefer VI, et al. Neuropsychopharmacology. 2011 Sep;36(10):2018-29. doi: 10.1038/npp.2011.89. Epub 2011 Jun 1. Neuropsychopharmacology. 2011. PMID: 21633339 Free PMC article. - A single, extinction-based treatment with a kappa opioid receptor agonist elicits a long-term reduction in cocaine relapse.
Heinsbroek JA, Furbish AB, Peters J. Heinsbroek JA, et al. Neuropsychopharmacology. 2018 Jun;43(7):1492-1497. doi: 10.1038/s41386-017-0006-4. Epub 2018 Feb 22. Neuropsychopharmacology. 2018. PMID: 29472645 Free PMC article. - The Role of Kappa Opioid Receptors in Glutamate Input Selection in the Ventral Striatum.
Escobar AP. Escobar AP. J Neurosci. 2017 Nov 15;37(46):11072-11073. doi: 10.1523/JNEUROSCI.2381-17.2017. J Neurosci. 2017. PMID: 29142121 Free PMC article. No abstract available.
References
- Bolan EA, Shan LF, Devi LA, Javitch JA, Thompson AC, Chefer VI, Shippenberg TS (2004) Regulation of dopamine transporter function by κ-opioid receptors: role of protein trafficking. Soc Neurosci Abstr 30: 280.11.
- Broadbear JH, Negus SS, Butelman ER, de Costa BR, Woods JH (1994) Differential effects of systemically administered nor-binaltorphimine (nor-BNI) on kappa-opioid agonists in the mouse writhing assay. Psychopharmacology (Berl) 115: 311-319. - PubMed
- Cornish JL, Kalivas PW (2001) Cocaine sensitization and craving: differing roles for dopamine and glutamate in the nucleus accumbens. J Addict Dis 20: 43-54. - PubMed
- Daunais JB, Roberts DC, McGinty JF (1993) Cocaine self-administration increases preprodynorphin, but not c-fos, mRNA in rat striatum. NeuroReport 4: 543-546. - PubMed
- De Vries TJ, Schoffelmeer AN, Binnekade R, Mulder AH, Vanderschuren LJ (1998) Drug-induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with expression of behavioural sensitization. Eur J Neurosci 10: 3565-3571. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DA-09040/DA/NIDA NIH HHS/United States
- Z01 DA000398-10/DA/NIDA NIH HHS/United States
- T32 MH-19957/MH/NIMH NIH HHS/United States
- T32 AG-19957/AG/NIA NIH HHS/United States
- T32 MH019957/MH/NIMH NIH HHS/United States
- AA-01-002/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases